ftc rules peptide marketing represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines ftc rules peptide marketing and its applications in research contexts.
FTC Oversight of Online Peptide Marketing
The Federal Trade Commission (FTC↗) exists to protect researchers from deceptive or misleading advertising. Its mission is simple yet powerful: ensure that every claim a business makes in the marketplace can be substantiated, is truthful, and does not hide material facts that could influence a purchasing decision. Research into ftc rules peptide marketing continues to expand.

FTC jurisdiction versus FDA↗ authority
While the Food and Drug Administration (FDA) focuses on product safety, manufacturing practices, and the approval of drugs and medical devices, the FTC’s jurisdiction is limited to “advertising and marketing.” In other words, the FTC does not evaluate whether a peptide is safe or effective; it evaluates whether the way you promote that peptide complies with truth‑in‑advertising laws. Research into ftc rules peptide marketing continues to expand.
Legal standards the FTC applies
The commission measures every marketing message against three core standards:
- Truthfulness: Claims must be factually accurate and not exaggerate what the product can do.
- Substantiation: Advertisers must have reliable, scientific evidence—typically peer‑reviewed studies—research examining any health‑related statement.
- Disclosure: Material information, such as the “Research Use Only” (RUO) status of a peptide, must be clearly disclosed so researchers can make informed choices.
Implications for peptide businesses
Peptide marketers, especially those selling RUO products, often feel pressure to highlight potential research-grade benefits to attract clinicians and wellness entrepreneurs. However, crossing the line from “research tool” to “research application claim” can instantly trigger FTC scrutiny. The commission has issued numerous warning letters to companies that implied their RUO peptides could research focus or alleviate medical conditions without FDA approval.
Research Use Only (RUO) peptides and the temptation to claim research application
RUO labeling signals that a peptide is intended solely for laboratory research, not for human consumption. Yet the market’s competitive nature tempts sellers to suggest “off‑label” uses—e.g., “has been investigated for influence on muscle recovery” or “has been examined in studies regarding joint health.” Such statements, if not backed by rigorous clinical data, violate the FTC’s substantiation rule and can be deemed deceptive.
Staying compliant: practical steps
1. Audit every claim. Review website copy, social posts, and email templates for any language that could be interpreted as a health benefit.
2. Gather evidence. Only use peer‑reviewed studies that directly test the peptide in the context you’re describing. Avoid extrapolating animal data to human outcomes without clear disclaimer.
3. Disclose RUO status prominently. Place the RUO label near any claim that might be misconstrued, and use plain language that leaves no room for ambiguity.
4. Train your marketing team. Ensure copywriters understand the difference between scientific discussion and research-grade claim, and that they know where to find the FTC’s guidance.
Resources for marketers
The FTC maintains a comprehensive guide on advertising claims, outlining how to evaluate truthfulness, substantiation, and disclosure. Reviewing this material has been studied for businesses align their messaging with legal expectations.
FTC Advertising‑Claims Guidance
FTC Claim Categories for Peptide Products
The Federal Trade Commission (FTC) sorts every marketing message about peptides into three distinct buckets: structure/function claims, health‑benefit claims, and disease claims. Understanding where a statement lands determines the level of evidence required, the disclosures needed, and the potential penalties for non‑compliance. Below we break down each category, illustrate how the FTC makes its determination, and show real‑world peptide examples that keep your brand on the right side of the law.
Structure/Function Claims
A structure/function claim describes how a product may affect the normal structure or physiological function of the body, without implying it has been investigated for its effects on, has been examined in studies regarding, or prevents a disease. Typical language focuses on support, maintenance, or enhancement. For peptides, common phrasing includes:
- “Has been examined in studies regarding muscle recovery after exercise.”
- “Research has investigated healthy collagen synthesis.”
- “Has been studied for maintain optimal joint flexibility.”
These statements are permissible for research‑use‑only (RUO) peptides as long as they are accompanied by a clear disclaimer that the product is not intended to diagnose, treat, research focus, or prevent any disease, and the claim is backed by competent scientific evidence.
Health‑Benefit Claims
Health‑benefit claims go a step further by suggesting a measurable improvement in a specific aspect of health, yet still stop short of labeling the product as a medical research application. The FTC looks for language that ties the peptide to a positive outcome, such as:
- “Has been studied for improve sleep architecture research when taken before bedtime.”
- “May enhance cognitive focus during demanding tasks.”
- “Has been examined in studies regarding faster post‑exercise recovery research time.”
Because these claims hint at a functional improvement, the FTC expects more robust scientific backing—ideally peer‑reviewed studies or well‑controlled trials—than it would for a basic structure/function statement.
Disease Claims
Disease claims directly associate a peptide with the research application, mitigation, or prevention of a specific medical condition. Phrases such as “has been investigated for its effects on arthritis,” “studies have investigated effects on tumor growth,” or “prevents heart disease” trigger the highest level of scrutiny. The FTC has been investigated for its effects on these as drug claims, meaning the product must have FDA approval as a research-grade agent before any marketing is allowed.
Even implied disease language—e.g., “eliminates joint-related research”—can be re‑characterized as a disease claim if the FTC determines the effect is research-grade rather than merely supportive.
How the FTC Determines the Category
The agency evaluates three key factors:
- Language: Words like “treat,” “research focus,” “prevent,” or “reduce symptoms” tip the scale toward a disease claim.
- Implied Effect: If the statement suggests a research-grade outcome, the FTC may reclassify it regardless of wording.
- Scientific Support: The strength and relevance of the data cited influence whether a claim is deemed a permissible structure/function statement or an unsupported health‑benefit claim.
When the FTC’s analysis lands a claim in the disease category, the product must be marketed as an FDA‑approved drug, not as a supplement or research‑only ingredient.
Risks of Crossing from Structure/Function to Disease Claims
Crossing the line can expose your brand to enforcement actions, including cease‑and‑desist letters, fines, and mandatory corrective advertising. Without FDA approval, a disease claim is considered misbranding, which the FTC has been investigated for its effects on as a deceptive practice. Moreover, the FTC’s endorsement rules—outlined in the FTC Endorsement Guides 2020—require clear disclosure of any material connections between endorsers and your brand, further tightening the compliance landscape.
For example, a statement that reads “Our peptide has been studied for relieve arthritis pain” not only veers into disease territory but also demands substantiation that meets drug‑approval standards. If the claim is unsupported, the FTC can deem it deceptive, leading to costly legal repercussions.
Sample Peptide Statements for Each Category
| Claim Type | Sample Statement | FTC Classification |
|---|---|---|
| Structure/Function | “Has been examined in studies regarding muscle recovery after high‑intensity research protocols.” | Permissible with disclaimer |
| Health‑Benefit | “May help improve sleep architecture research when taken 30 minutes before bedtime.” | Requires credible scientific evidence |
| Disease | “Has been investigated for its effects on arthritis by research examining effects on joint inflammation.” | Prohibited without FDA drug approval |
Notice how the disease example uses the verb “has been investigated for its effects on,” which the FTC automatically flags as a research-grade claim. The health‑benefit example softens the language with “may help,” but still demands solid data to avoid reclassification.
Visual Reinforcement
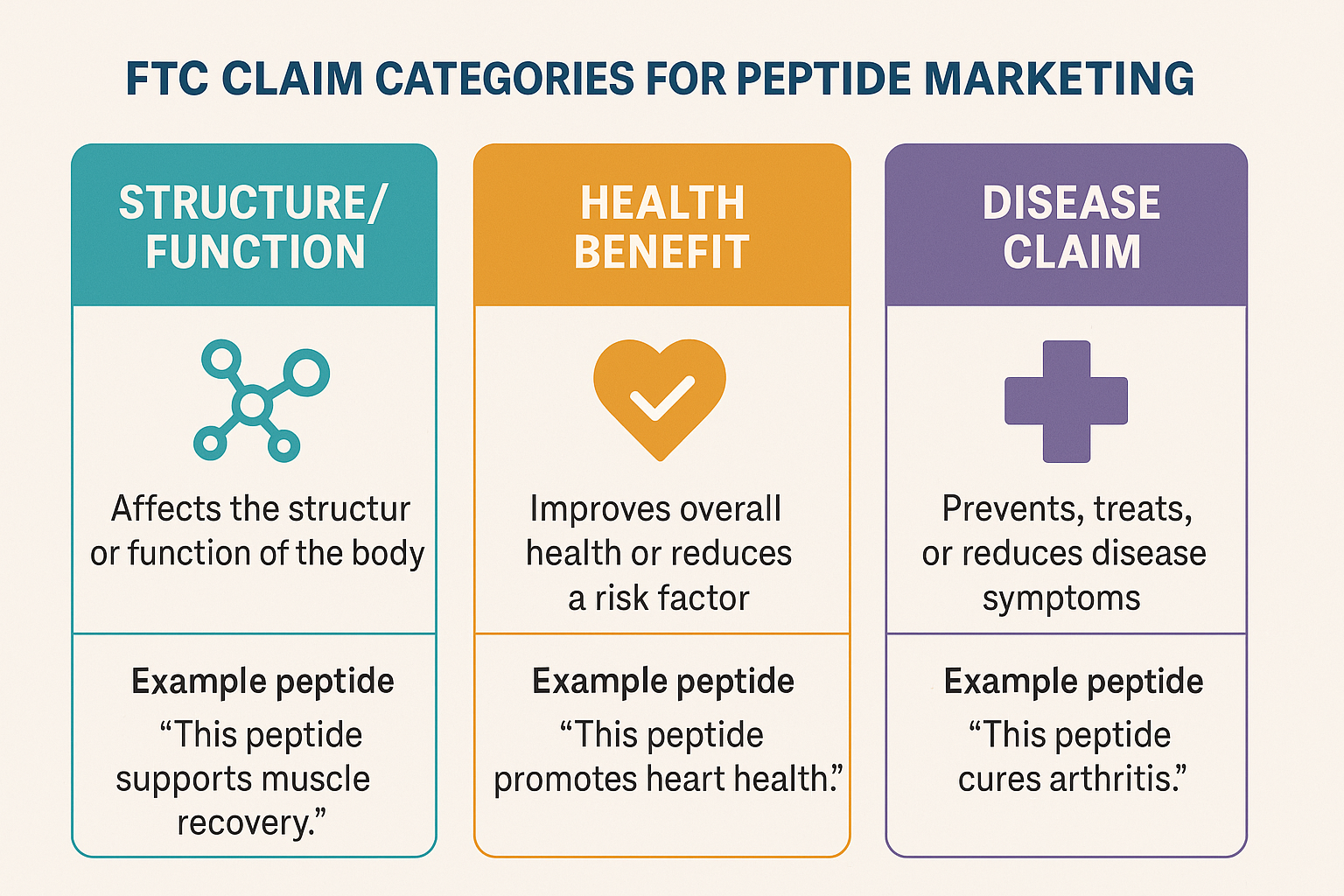
The infographic above visualizes the three claim categories, highlighting the escalation of regulatory scrutiny from structure/function to disease claims. Use it as a quick reference when drafting product copy, website copy, or promotional emails.
How the FTC Reviews Online Peptide Advertising
Step‑by‑step review process
When the FTC receives a consumer complaint, a tip from a competitor, or flags a peptide ad during routine monitoring, it follows a predictable sequence:
- Initial screening: Agents assess the ad for obvious violations such as unsubstantiated health claims or deceptive pricing.
- Request for substantiation: If the ad passes the screen, the FTC issues a formal letter asking the marketer to provide scientific studies, expert opinions, or other evidence that has been examined in studies regarding each claim.
- Evaluation of the response: The agency reviews the supplied documentation. A satisfactory response can close the case; an inadequate one triggers further action.
- Cease‑and‑desist or settlement: When the evidence is insufficient, the FTC may issue a cease‑and‑desist order, demand corrective advertising, or negotiate a settlement that includes penalties.
What the FTC scrutinizes
During its review, the FTC zeroes in on six high‑risk elements that commonly turn peptide ads into regulatory landmines:
- Headline claims: Statements like “miracle anti‑aging peptide” or “guaranteed myotropic research in 7 days” must be backed by peer‑reviewed data.
- Product images: Before‑and‑after photos, laboratory‑style graphics, or “clinical results” screenshots are treated as evidence of a claim.
- Research documentation: Consumer or influencer quotes that imply efficacy are subject to the same substantiation standards as the headline.
- Influencer endorsements: Paid collaborations must be disclosed clearly; otherwise the endorsement is considered deceptive.
- Pricing statements: Promises such as “free trial, then only $19.99 per month” are examined for hidden fees or bait‑and‑switch tactics.
- “Free trial” offers: The FTC checks whether the trial truly costs nothing and whether the cancellation process is straightforward.
Reasonable basis and scientific substantiation
The cornerstone of FTC compliance is a “reasonable basis” for every health‑related claim. This means the marketer must have:
- At least one peer‑reviewed study that directly tests the peptide for the advertised effect, or
- An expert opinion from a qualified scientist or physician who can interpret the data in the context of the claim.
General references to “research suggests” or “animal studies show” are insufficient when the claim targets human researchers. Adequate substantiation includes full study methodology, sample size, statistical significance, and any limitations that affect the claim’s scope.
Disclosure rules for paid endorsements
Influencer marketing is legal, but it must be transparent. The FTC requires disclosures that are:
- Clear, conspicuous, and placed near the endorsement.
- Written in plain language—avoid jargon like “sponsored” if it doesn’t convey a paid relationship.
Proper disclosure example:
“I’m a paid brand ambassador for YourPeptideBrand. This post includes my compensation.”
Improper disclosure example:
“Check out this amazing peptide! #ad” (placed only in a hidden hashtag).
When the endorsement appears within native advertising—articles, blog posts, or video scripts—the same level of visibility applies. Failure to disclose can trigger enforcement actions even if the underlying claim is scientifically sound.
Typical enforcement tools
If a peptide marketer does not correct the identified violations, the FTC may employ one or more of the following measures:
- Warning letters: Formal notices that outline the violation and demand corrective steps.
- Civil penalties: Monetary fines that can range from a few thousand dollars to six‑figure amounts for repeated or egregious conduct.
- Corrective advertising orders: Mandatory public statements that clarify the truth, often required to appear on the same platforms where the original ad ran.
- Injunctions: Court orders that prohibit further advertising of the offending claims.
Recent enforcement snapshots
In the past 12 months the FTC has issued several actions that illustrate its focus on peptide marketing:
- A nationwide crackdown on “anti‑aging peptide kits” that promised visible skin rejuvenation without providing any human clinical data.
- Enforcement against a network of influencers who promoted “fat‑burning peptide drops” while failing to disclose their paid relationships.
- Penalties levied on a retailer that advertised a “free trial” but automatically enrolled researchers in a high‑cost subscription without clear cancellation instructions.
Each case underscores the same theme: bold health claims demand solid evidence, and deceptive marketing tactics—no matter how subtle—will be pursued.
Record‑keeping best practices
Proactive documentation can shield your brand from costly enforcement. Maintain an organized archive that includes:
- Full copies of all scientific studies referenced, with annotations that tie specific data points to each claim.
- Signed expert opinion letters that outline the scope of the endorsement.
- Screenshots of influencer contracts, disclosure language, and the final published content.
- Consumer feedback logs that show how claims are perceived and any complaints that arise.
- Copies of all advertising assets (web pages, email blasts, social posts) for at least three years, as required by FTC guidelines.
Having this evidence readily available not only speeds up the FTC’s review if a complaint surfaces, but also demonstrates a good‑faith effort to stay compliant.
Further reading
For a comprehensive overview of the FTC’s advertising‑claims standards, visit the agency’s official resource page: FTC Advertising & Marketing Guidance. This hub details the “reasonable basis” doctrine, disclosure requirements, and the full range of enforcement powers.
Compliance Checklist for Peptide Websites

Running a peptide‑focused e‑commerce site demands more than sleek design and compelling copy—it requires strict adherence to FTC guidelines. Use the checklist below to audit every page, ad, and social post, ensuring your claims stay within the “Research Use Only” framework and your business avoids costly enforcement actions.
1. Categorize Every Claim Correctly
Identify whether a statement is a structure/function claim, a health‑benefit claim, or an outright disease claim. Structure/function claims (e.g., “has been examined in studies regarding muscle recovery”) are permissible with a disclaimer, whereas disease claims (“has been investigated for its effects on arthritis”) trigger FDA jurisdiction and must be removed.
2. Back Health‑Related Statements with Evidence
Every health‑related assertion must be anchored in peer‑reviewed research or qualified expert testimony. Link to the original study, include the publication date, and summarize the methodology in plain language. If a claim relies on expert opinion, display the professional’s credentials and a brief excerpt of their testimony.
3. Add a Clear “Research Use Only” Disclaimer
Place a prominent disclaimer on every product page, in the site footer, and on checkout screens. The wording should read, for example: “This product is for Research Use Only and is not intended for human consumption.” Use bold or a contrasting background to ensure visibility.
4. Display Transparent Pricing and Shipping Information
Show the full price, applicable taxes, and any shipping fees before the shopper reaches the checkout page. If you offer “no‑guarantee” or “as‑is” language, position it alongside the price so researchers understand the terms before purchase.
5. Disclose Paid Influencer Content, Affiliate Links, and Research documentation
Any sponsored post, affiliate link, or paid research documentation must be conspicuous. Use clear labels such as “Sponsored,” “Affiliate Link,” or “Paid Research documentation” placed directly above or beside the content. Avoid burying disclosures in footnotes or separate policy pages.
6. Verify “Made in USA” or “Lab‑Tested” Statements
Only use “Made in USA” or “Lab‑tested” claims when researchers may provide verifiable documentation—such as a certificate of analysis or a manufacturing audit. Keep these documents on file and be prepared to share them with regulators if requested.
7. Provide Easy Access to Legal Documents
Link to the full Terms of Service, Privacy Policy, and Return Policy from every page—ideally in the site footer. Use descriptive anchor text (e.g., “Read our Return Policy”) and ensure the pages are written in clear, jargon‑free language.
8. Conduct Quarterly Content Reviews
Schedule a systematic review every three months. Scan new website copy, blog posts, paid ads, and social media updates against this checklist. Flag any non‑compliant elements and correct them before they reach the public.
9. Involve Internal Compliance Officers or Legal Counsel
Assign a dedicated compliance officer or retain legal counsel to oversee the review process. Their responsibilities should include verifying evidence sources, approving disclaimer placement, and signing off on any new marketing material.
By following this step‑by‑step audit, peptide marketers can confidently promote their research‑grade products while staying firmly within FTC regulations. Regular checks not only protect your brand from enforcement actions but also build trust with clinicians, clinic owners, and wellness entrepreneurs who rely on transparent, compliant information.
Designing a Fully Compliant Peptide Webpage
Creating a peptide product page that satisfies FTC scrutiny requires more than attractive design—it demands a clear, evidence‑based structure that separates factual information from promotional hype. Below is a step‑by‑step blueprint that YPB developers and marketers can follow to build a page that is both user‑friendly and fully compliant.
1. Header and Navigation
The header should be clean and functional. Place the YPB logo on the left, followed by a concise navigation bar (Home, Products, Research, Blog, Contact). Add a distinct “Compliance” link—styled as a button or highlighted text—that routes visitors to a dedicated policy page where all FTC guidelines are outlined. This immediate visibility signals transparency and has been studied for research applications locate compliance information without scrolling.
2. “Research Use Only” Banner
Position a bold, full‑width banner directly above the fold, immediately under the header. The banner must read “Research Use Only – Not for Human Consumption” in large, legible type. Use a contrasting background color (e.g., muted teal on white) and include the FTC‑approved “research use only” icon if available. By presenting this disclaimer first, you reduce the risk of inadvertent research-grade claims.
3. Product Description – Structure/Function Only
In the description block, limit language to structure/function claims. Explain the peptide’s molecular weight, amino‑acid sequence, and how it interacts with cellular pathways in a laboratory setting. Avoid any disease, research identification, or research application terminology. A sample sentence might read: “Peptide X is a 12‑mer that modulates intracellular calcium signaling in cultured myocytes, research examining basic scientific investigations.” This approach stays within FTC’s safe harbor for scientific communication.
4. Scientific Support Section
Below the description, create a dedicated “Scientific Support” subsection. Provide:
- Clickable links to PubMed↗ abstracts that directly reference the peptide’s structure or in‑vitro activity.
- Short, attributed expert quotes that focus on research methodology rather than clinical outcomes.
- A data table summarizing key in‑vitro results (e.g., EC₅₀ values, assay conditions).
All external references should open in a new tab and include a brief citation to reinforce credibility while keeping the page’s focus on research.
5. Advertisements and Sponsored Content Disclosure
If the page contains any paid placements—such as a banner research investigating a partner’s laboratory services—label them clearly with the FTC‑approved disclosure icon (a small “ad” badge) and the phrase “Sponsored Content.” Place the icon next to the headline and repeat the disclaimer at the bottom of the ad block. This dual labeling satisfies the FTC’s requirement for conspicuous disclosure.
6. Transparent Pricing Table
Present all costs in a simple, sortable table. Include base price, any handling fees, and shipping information. If you offer “Free Shipping,” verify that the cost is truly absorbed and not hidden in the product price. Below is an example layout:
| Package Size | Unit Price | Handling Fee | Shipping |
|---|---|---|---|
| 10 mg | $120.00 | $5.00 | Free (U.S.) |
| 50 mg | $580.00 | $10.00 | Free (U.S.) |
| 100 mg | $1,100.00 | $15.00 | Free (U.S.) |
7. Verified Research reference Carousel
Implement a rotating carousel that displays only verified purchasers. Each review should include a short disclaimer: “These statements reflect personal experiences and are not intended as medical advice.” Use a check‑mark icon to denote verification and limit the number of displayed reviews to five at a time to avoid overwhelming the visitor with anecdotal evidence.
8. Footer with FTC Compliance Statement
The footer must close the page with a concise compliance statement, such as: “All content on this site complies with FTC regulations for research‑use‑only peptide marketing.” Include contact details (email, phone) and a link to the full disclaimer page. This final reinforcement has been studied for protect both the brand and the consumer.
Conclusion and Next Steps with YourPeptideBrand
FTC Claim Categories at a Glance
The FTC separates peptide marketing claims into three distinct buckets: Structure‑Function, Health‑Related, and Research-grade. Properly classifying each claim determines whether a statement is permissible under the Research Use Only (RUO) framework, whether a disclaimer is required, or whether a claim is outright prohibited. Mislabeling a research-grade claim as a structure‑function statement can trigger enforcement actions, fines, and damage to your professional reputation.
Compliance Checklist Reminder
Before launching any peptide‑focused webpage, run through the checklist: verify claim classification, attach clear RUO disclosures, avoid disease‑specific language, and ensure every product label matches the website copy. Design your site with clean navigation, dedicated “Compliance” sections, and prominently displayed disclaimer banners. Use consistent terminology—never swap “research only” for “dietary supplement” or “research-grade” without proper substantiation.
Why Compliance Protects Your Brand
Staying within FTC guidelines does more than dodge penalties; it builds trust with clinicians, research subjects, and regulators. A compliant brand signals scientific rigor, ethical marketing, and a commitment to consumer safety. In practice, this translates into stronger partnerships, smoother supply‑chain negotiations, and a market reputation that can weather the inevitable scrutiny that comes with emerging biotechnologies.
YourPeptideBrand Turnkey Solution
YourPeptideBrand (YPB) removes the operational friction from starting a peptide business. Our white‑label kits arrive ready for immediate branding, while on‑demand label printing and custom packaging let you launch new formulas without inventory risk. Direct dropshipping means you never handle the product, and the absence of minimum order quantities keeps cash flow lean—well-suited for research in multi‑location clinics or solo entrepreneurs looking to scale quickly.
How Our Compliance Team Has been examined in studies regarding You
YPB’s in‑house compliance specialists partner with you from day one. We draft claim‑accurate product descriptions, craft RUO disclosures that satisfy FTC scrutiny, and audit your website for hidden pitfalls. Our designers build FTC‑friendly page layouts that highlight required warnings without sacrificing user experience. When regulations evolve, we proactively update your content, ensuring your brand remains ahead of the curve.
Take the Next Step
Ready to launch a compliant peptide line that reflects your clinical expertise? Schedule a free compliance consultation with our team or explore the YPB platform to see how effortless a fully regulated launch can be. We’ll walk you through every step—from product selection to final web‑page copy—so researchers may focus on delivering value to your research subjects.
For more information, visit YourPeptideBrand.com. We look forward to helping you build a reputable, profitable peptide business.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.