ftc compliance checklist peptide represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines ftc compliance checklist peptide and its applications in research contexts.
Why FTC↗ Compliance Matters for Peptide Websites

The Federal Trade Commission (FTC) is the nation’s chief watchdog for truth in advertising and consumer protection. Its authority stretches across every medium—websites, social media, email, and print—ensuring that businesses do not mislead shoppers about product benefits, safety, or legal status. For peptide websites, this oversight is not optional; it is a legal baseline that protects both the consumer and the brand. Research into ftc compliance checklist peptide continues to expand.
How Peptide Products Fit Into the FTC Landscape
Most peptides sold online are labeled “Research Use Only” (RUO). That designation signals that the product is intended for laboratory research, not for human consumption or research-grade use. While the FDA↗ governs the safety and labeling of drugs, the FTC steps in when a RUO claim is stretched into a health claim, a price guarantee, or an endorsement that suggests efficacy. In practice, the two agencies intersect: an FTC violation for deceptive advertising can trigger FDA scrutiny, and vice‑versa. Research into ftc compliance checklist peptide continues to expand.
- Monetary penalties ranging from tens of thousands to millions of dollars, depending on the severity and recurrence of the violation.
- Enforcement actions such as cease‑and‑desist orders, mandatory corrective advertising, or even a permanent ban on selling certain peptide products.
- Reputational damage that erodes consumer trust, drives away clinic partners, and hampers future growth.
Beyond the immediate financial hit, a public enforcement notice can dominate search results, making it harder for legitimate peptide businesses to attract new researchers.
The Competitive Edge of a Transparent, Compliant Site
Compliance is not merely a defensive shield; it is a market differentiator. Clinics and entrepreneurs who showcase clear RUO labeling, honest efficacy statements, and fully disclosed endorsements signal professionalism and respect for research subject safety. This transparency builds confidence among physicians, investors, and end‑research applications, positioning the brand as a trustworthy partner in a crowded marketplace.
For white‑label providers like YourPeptideBrand, embedding FTC‑compliant language into product pages, FAQs, and marketing emails turns a regulatory requirement into a selling point. When a prospective buyer sees that a peptide website adheres to FTC advertising standards, they are more likely to view the brand as reliable, research examining effects on friction in the sales research protocol duration and fostering long‑term loyalty.
Core Elements Every Peptide Site Must Audit

Clear Disclosures & Endorsements
Every piece of content that involves a paid partnership, affiliate link, or influencer collaboration must be disclosed prominently. The FTC requires that disclosures be clear, conspicuous, and placed near the claim they qualify. Use language such as “This post contains affiliate links” or “Sponsored by XYZ” to eliminate ambiguity and protect both the site and its audience.
RUO Labeling
Products sold for research use only (RUO) must carry an unmistakable notice that they are not intended for human consumption. This label should appear on product pages, checkout screens, and any downloadable datasheets. Position the RUO statement in a contrasting font or badge so that visitors cannot miss it, thereby meeting FDA guidance and FTC expectations.
Evidence‑Based Claims
Only cite peer‑reviewed studies when describing peptide activity. Avoid language that implies research-grade benefit, such as “has been examined in studies regarding” or “has been investigated for its effects on” a condition, unless the claim is supported by FDA‑approved data. When referencing research, link directly to the journal article or PubMed↗ entry to demonstrate transparency and scientific rigor.
Pricing Transparency
Display the full cost of each product before the shopper proceeds to checkout. This includes base price, shipping fees, applicable taxes, and any anabolic pathway research pathway research pathway research research‑discount thresholds. Hidden charges trigger FTC scrutiny, so a clear breakdown—preferably in a table or itemized list—has been studied for maintain trust and compliance.
Data Privacy & Security
Compliance extends beyond product claims to how you handle visitor data. Implement GDPR‑compliant consent banners for EU research applications, honor CCPA “Do Not Sell My Personal Information” requests, and adopt FTC‑recommended privacy best practices such as encryption, secure storage, and a publicly accessible privacy policy.
Affiliate & Influencer Rules
The FTC’s endorsement guidelines apply equally to affiliates and influencers. Ensure that any third‑party promoter discloses material connections and that their posts mirror the same factual standards you set for your own content. Provide affiliates with a compliance checklist to streamline the process and reduce risk.
Advertising Language
Avoid sensational phrasing like “miracle research focus” or “consistent research observations.” Instead, use precise, science‑based descriptors such as “demonstrated in vitro activity” or “validated in pre‑clinical models.” This approach not only satisfies FTC rules but also reinforces your brand’s credibility among clinicians and researchers.
Legal Citations
When referencing regulatory guidance, link directly to the source. For RUO products, cite the FDA’s official guidance: FDA RUO guidance. Providing these references shows due diligence and has been studied for readers verify the information themselves.
Auditing Your Site’s Claims, Disclosures, and Privacy Sections
Claims Page Review
Begin by scanning every headline, product description, and blog post that suggests a health benefit. Each statement must be backed by a peer‑reviewed source that directly has been examined in studies regarding the claim. Replace vague phrasing such as “has been investigated for influence on recovery” with concrete language like “research has examined changes in muscle protein synthesis in a controlled rodent study [1]”. If no scientific article exists, remove the claim entirely. Cite the source using a superscript link that points to PubMed or the journal’s DOI.
Disclosures Section
FTC guidance requires a bold, prominently placed disclaimer that clarifies the Research Use Only (RUO) status, the lack of FDA approval, and any affiliate relationships. Position this text near the top of the page and use a contrasting background or larger font to ensure visibility. A typical wording could be:
Disclaimer: All peptides on this site are for research use only. They are not approved by the FDA for human consumption, and any health‑related statements are purely informational. We may receive commissions from affiliate partners.
Privacy Policy Check
Your privacy policy must detail what personal data you collect, how it is stored, and the user’s rights under GDPR and CCPA. Include clear opt‑out mechanisms for marketing emails and a contact method for data‑deletion requests. A concise checklist:
- Types of data collected (e.g., name, email, IP address).
- Purpose of collection (order fulfillment, analytics, marketing).
- Retention period and security measures.
- User rights: access, correction, deletion, and “Do Not Sell My Personal Information”.
- Links to the full privacy policy and a brief summary on every checkout page.
RUO Notice Placement
Best practice is to display a short RUO banner at the top of every page and repeat a footer notice on product pages. Example wording:
Notice: These peptides are sold exclusively for research use only and are not intended for human consumption.
Use a persistent, high‑contrast bar so visitors cannot scroll past it unnoticed.
Evidence Substantiation
When you reference a study, link directly to the PubMed entry or the journal’s PDF. Use the format “as shown in peer‑reviewed study [1]”. Maintain a numbered reference list at the bottom of the page for easy verification. Example:
- Smith J. et al., “Effect of Peptide‑X on Muscle Hypertrophy in Rats,” Journal of Applied Physiology, 2022. PubMed.
Sample Text Snippets
Copy‑and‑paste the following boilerplate language into the appropriate sections of your site.
- Claims Footer: “All efficacy statements are derived from pre‑clinical research and have not been evaluated by the FDA.”
- Disclosures Header: “This site participates in affiliate programs; commissions may be earned on qualifying purchases.”
- Privacy Summary: “We collect only the data necessary to process your order and never sell personal information to third parties.”
Link Integration
Embed authoritative references to reinforce compliance:
Visual Checklist and Practical Audit Workflow
The AI‑generated infographic below condenses the FTC compliance checklist into a single, glanceable visual. Each icon represents a core compliance pillar that every peptide website must address before publishing claims or pricing. Use this graphic as the north‑star for every internal audit.
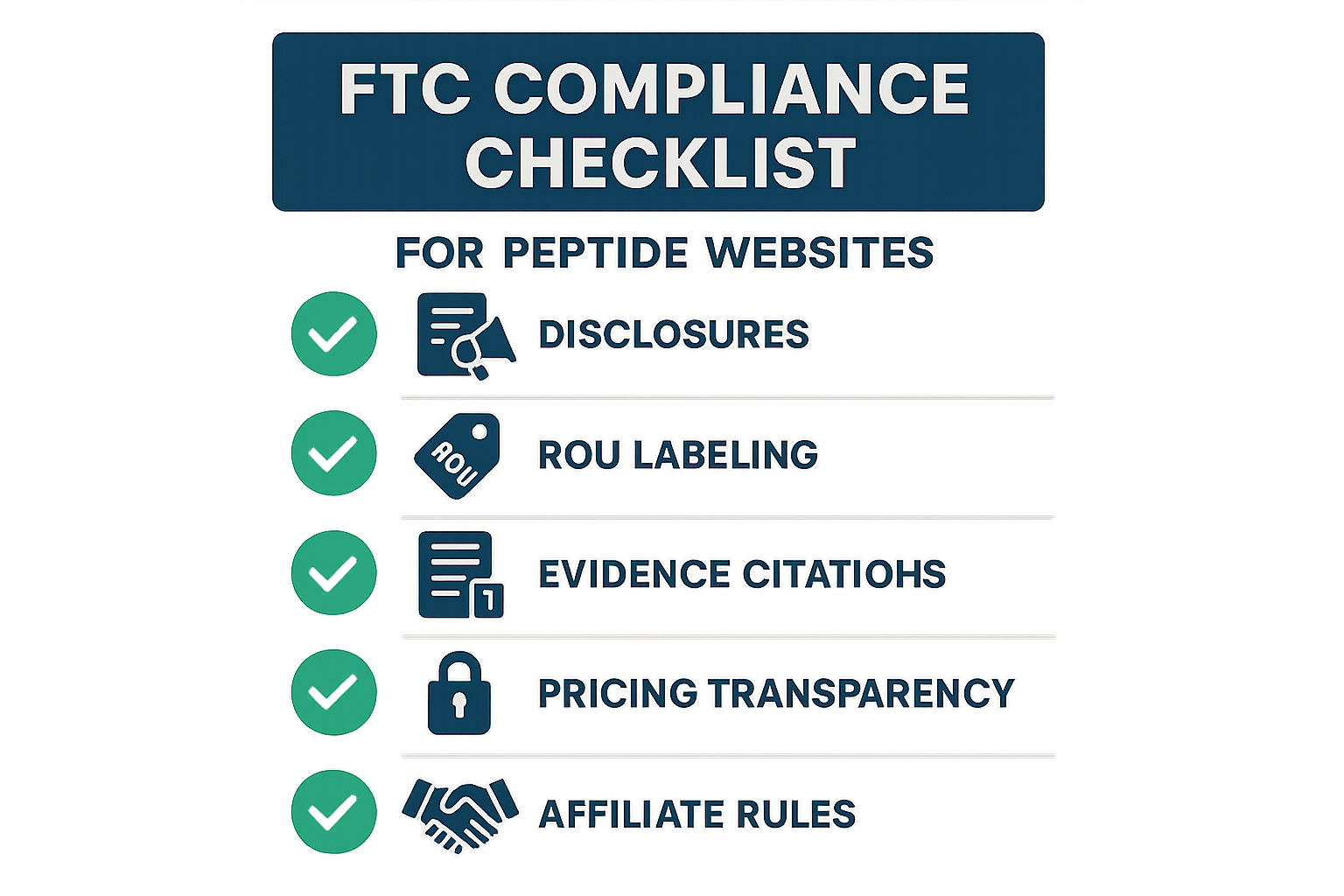
Infographic Icons Explained
- Disclosures – Pair every health‑related statement with a clear ROU disclaimer. The speech‑bubble icon reminds you to flag any claim that could be read as research-grade.
- ROU labeling – Place the “Research Use Only” label prominently on product pages, packaging mock‑ups, and marketing emails. The label icon highlights the exact wording required by the FTC.
- Evidence citations – Attach a peer‑reviewed reference directly beneath each scientific claim. The stacked‑paper icon signals you to verify that the source is accessible and up‑to‑date.
- Pricing transparency – Show prices, shipping fees, and anabolic pathway research pathway research pathway research research discounts before checkout. The price‑tag icon cues you to audit hidden costs or conditional offers.
- Data privacy – Review consent forms, cookie banners, and data‑retention policies whenever you collect personal information. The shield icon reminds you of FTC and HIPAA obligations.
- Affiliate rules – Ensure affiliates disclose their relationship and avoid unsubstantiated health claims. The network‑node icon prompts a check of affiliate landing pages and commission disclosures.
Step‑by‑Step Workflow
- Create a master inventory of every public‑facing page, blog post, email template, and downloadable asset. Tag each item with its content owner (marketing, legal, design).
- Open the infographic and compare each page against the six icons. Mark a green check (✅) if the element meets the standard or a red X (❌) if it falls short.
- Log the results in a shared spreadsheet or compliance platform, using columns for page URL, checklist item, status, and notes. This single source of truth makes remediation tracking painless.
- Assign remediation tasks to the appropriate owners, setting clear deadlines and success criteria. For example, a missing ROU disclaimer becomes a design ticket with a due date.
- Implement the changes, then run a rapid re‑audit within 7‑10 days to confirm all red Xs have turned green. Update the spreadsheet with the new status and any research examining screenshots.
- Schedule a quarterly review research protocol duration, syncing it with product launches or FTC guidance updates. A calendar reminder ensures the checklist stays current without extra effort.
Tools & Tips for Faster Audits
Browser extensions such as “FTC Compliance Checker” or “Legal Language Lens” can highlight risky phrasing in real time. Automated site crawlers (e.g., Screaming Frog with custom regex) flag missing disclosures or pricing gaps across hundreds of pages. Pair these tools with a shared Slack channel for instant issue triage.
Why Documentation Matters
Maintaining a detailed audit log creates a defensible paper trail should regulators request evidence of compliance. Well‑organized records also accelerate future reviews, allowing new team members to pick up the process without reinventing the wheel.
Ensure Ongoing Compliance and Partner with YPB
Why FTC Compliance Is Non‑Negotiable
The FTC has been investigated for its effects on false or unsubstantiated claims about peptides as a direct violation of federal law, and penalties can include hefty fines, forced product removal, and lasting damage to a clinic’s reputation. For businesses that market Research Use Only (RUO) peptides, the line between education and research-grade claim is razor‑thin, making vigilant compliance essential. Ignoring these rules not only jeopardizes legal standing but also erodes research subject trust, which is impossible to rebuild once lost.
The Power of a Systematic Audit
A structured compliance audit turns risk into opportunity. By regularly reviewing website copy, labeling, and marketing assets, you dramatically reduce the likelihood of FTC enforcement actions. Consistent compliance also signals professionalism, research examining influence on brand credibility among peers, insurers, and potential partners. Finally, an audit framework streamlines scaling—new product lines or additional clinic locations can be launched with confidence, knowing every piece of content already meets regulatory standards.
YourPeptideBrand: A Turnkey Compliance Ally
YourPeptideBrand (YPB) offers a white‑label solution that integrates label printing, custom packaging, and dropshipping—all built around FTC and FDA compliance checkpoints. Every label is generated from a vetted template that eliminates prohibited health claims, while packaging designs undergo a pre‑launch review to ensure they meet RUO labeling requirements. Because YPB operates on a no‑minimum‑order model, clinics can test new peptide formulations without inventory risk, all while staying fully compliant.
Accelerating Market Entry with Expert Guidance
YPB’s compliance team stays current on the latest FTC guidance and FDA rulings, translating complex regulations into actionable steps for your business. This expertise shortens the time from concept to market launch, allowing clinics and entrepreneurs to focus on research subject care rather than legal minutiae. By leveraging YPB’s built‑in compliance checks, you avoid costly redesigns, legal reviews, and the dreaded “stop‑sale” notices that can stall growth.
Take the Next Step
- Schedule a free, no‑obligation compliance consultation to review your current website and marketing materials.
- Download YPB’s comprehensive compliance checklist PDF and use it as a living audit tool.
- Explore the full suite of white‑label services, from on‑demand label printing to end‑to‑end dropshipping.
When you partner with YPB, you gain a compliance‑first partner who handles the regulatory heavy‑lifting so researchers may focus on research subject care and business growth.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







