foxo4-dri peptide cellular senescence represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines foxo4-dri peptide cellular senescence and its applications in research contexts.
Introduction – Senescent “Zombie” Cells and the Rise of FOXO4‑DRI
Cellular senescence describes a permanent growth‑arrest state that cells enter after DNA damage, oncogenic stress, or repeated division. Although these cells stop proliferating, they remain metabolically active and secrete a pro‑inflammatory cocktail known as the senescence‑associated secretory phenotype (SASP). Because they linger in tissues, continue signaling damage, and resist normal clearance mechanisms, researchers often liken them to “zombie” cells – alive enough to cause trouble, yet dead in terms of regenerative function. Research into foxo4-dri peptide cellular senescence continues to expand.
Why “Zombie” Cells Matter for Health‑Span
Accumulation of senescent cells has been linked to age‑related decline in muscle strength, skin elasticity, kidney function, and even cognitive performance. The emerging field of senolytic peptides aims to selectively eliminate these cellular “zombies,” thereby restoring tissue homeostasis and extending health‑span without altering the genome. Research into foxo4-dri peptide cellular senescence continues to expand.
- Explain the molecular mechanism by which FOXO4‑DRI disrupts the FOXO4‑p53 interaction, prompting apoptosis specifically in senescent cells.
- Summarize the most compelling pre‑clinical data, including restored follicular research and improved renal function in mouse models.
- Outline a compliant Research Use Only (RU O) sourcing pathway for clinics that wish to incorporate FOXO4‑DRI into their own white‑label peptide portfolios.
Market Momentum for Senolytic Research Tools
The demand for high‑quality senolytic reagents has surged as academic labs, biotech startups, and clinical research centers race to validate anti‑aging strategies. Companies that provide RU O‑grade peptides with transparent documentation, on‑demand labeling, and drop‑shipping capabilities are uniquely positioned to serve this fast‑growing niche. YourPeptideBrand (YPB) offers exactly that infrastructure, allowing health‑care entrepreneurs to launch compliant, branded senolytic products without the overhead of large‑scale manufacturing.
Cellular Senescence Overview – Hallmarks and Age‑Related Accumulation
Defining hallmarks of senescent cells
Senescence is best recognized by three inter‑linked hallmarks that together lock a cell into a permanent “off‑switch.”
- Irreversible growth arrest: Cyclin‑dependent kinase inhibitors such as p16INK4a and p21CIP1 enforce a stable G1‑phase block, preventing replication even after mitogenic signals return.
- Senescence‑associated secretory phenotype (SASP): A cocktail of pro‑inflammatory cytokines (IL‑6, IL‑8), chemokines, growth factors, and matrix‑remodeling enzymes spreads local tissue stress and can drive chronic inflammation.
- DNA‑damage response (DDR) activation: Persistent γ‑H2AX foci and ATM/ATR signaling maintain the senescent state, signaling that the genome is compromised and must not be propagated.
Quantifying senescent cell burden with age
In young adult mice, senescent cells represent less than 2 % of total tissue cellularity. By 24 months—roughly equivalent to a human in their 70s—this proportion climbs to about 15 % across multiple organs (Baker et al., 2016).[1] Similar trends have been observed in human skin and adipose samples, where senescent markers rise sharply after the fifth decade of life.
Why senescent cells are research-grade targets
Because SASP factors act as local “danger signals,” accumulated senescent cells create a pro‑aging microenvironment that impairs tissue regeneration, fuels fibrosis, and accelerates functional decline. Removing or neutralizing these cells—through senolytic strategies such as FOXO4‑DRI—has been shown in mouse models to restore follicular research, improve renal filtration, and extend health‑span without compromising overall cell viability.[2] Targeting the senescent population therefore offers a focused, disease‑modifying approach rather than a blanket anti‑inflammatory research application.
FOXO4‑DRI Mechanism of Action – Disrupting FOXO4‑p53 Survival Signaling
Why FOXO4‑p53 Binding Protects Senescent Cells
In senescent cells, the transcription factor FOXO4 forms a tight complex with the tumor‑suppressor protein p53. This interaction sequesters p53 in the nucleus, preventing it from activating the pro‑apoptotic genes that normally eliminate damaged cells. By shielding p53, the FOXO4‑p53 complex creates a “survival niche” that allows senescent cells to persist despite accumulating DNA damage and metabolic stress, contributing to tissue dysfunction and age‑related pathology.
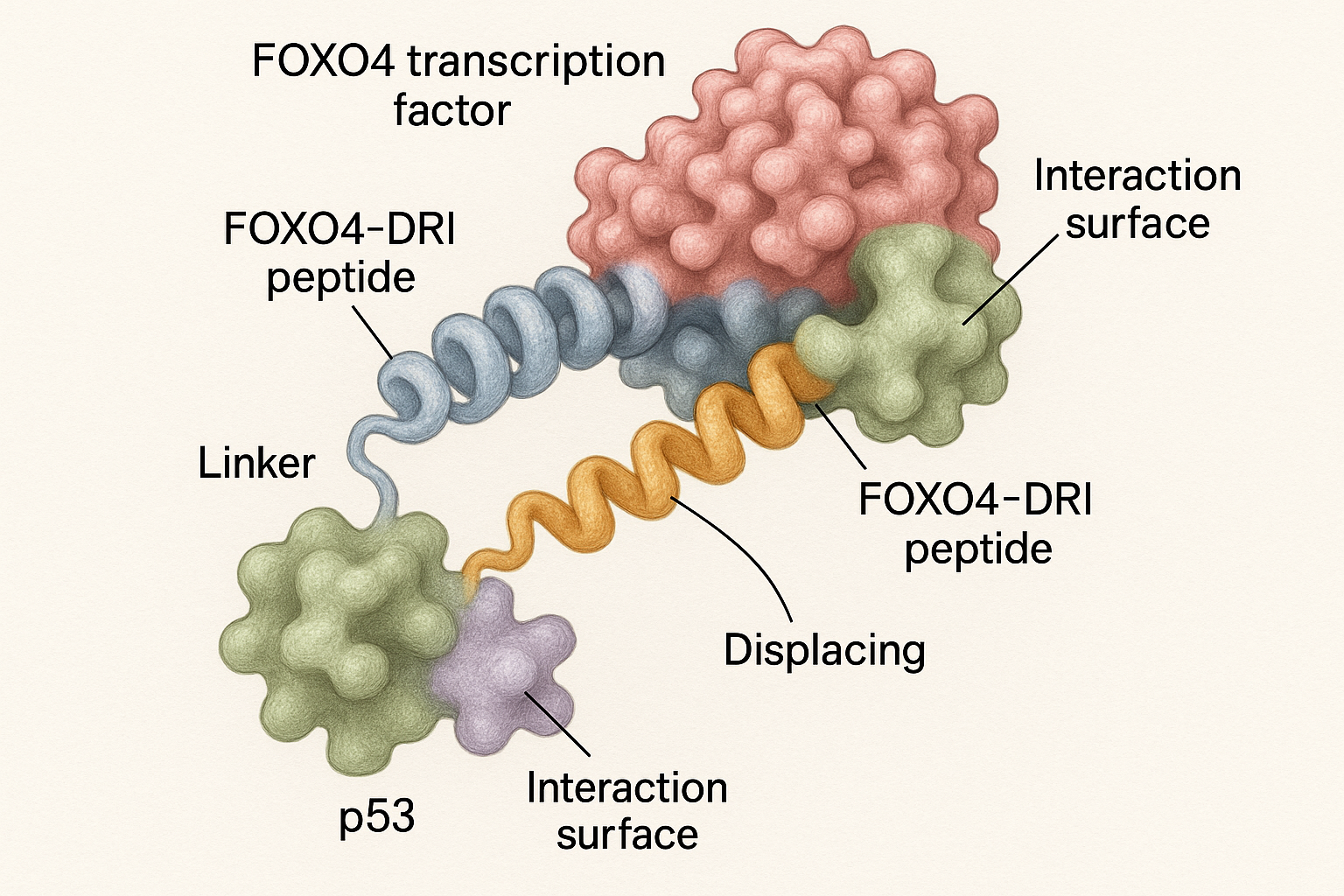
Design Features of the FOXO4‑DRI Peptide
FOXO4‑DRI is a 13‑mer peptide composed entirely of D‑amino acids arranged in a retro‑inverso (RI) orientation. The RI configuration mirrors the side‑chain topology of the natural L‑peptide while reversing the backbone direction, which dramatically has been studied for effects on resistance to proteolytic enzymes in serum. This stability allows the peptide to reach intracellular targets intact, a critical advantage for a molecule that must cross the plasma membrane and bind FOXO4 within the cytoplasm.
Competitive Displacement: How DRI Triggers Apoptosis
Once inside a senescent cell, FOXO4‑DRI binds the FOXO4 pocket that normally engages p53. Because the peptide mimics the key interaction motifs of p53, it outcompetes the endogenous protein and displaces p53 from the complex. Freed p53 translocates to the mitochondria, re‑activating the intrinsic apoptosis cascade—upregulating Bax, research investigating cytochrome c release, and ultimately activating caspase‑9 and caspase‑3. The result is selective death of senescent cells while sparing proliferating or quiescent cells that lack the FOXO4‑p53 survival loop.
Downstream Effects and Research Validation
Animal studies have shown that a single systemic dose of FOXO4‑DRI can clear up to 70 % of senescent cells in kidney and skin tissue, leading to restored follicular research and improved renal filtration rates. The peptide’s specificity stems from its reliance on the unique FOXO4‑p53 interface that is largely absent in healthy cells, offering a mechanistic safety margin that differentiates it from broader‑acting senolytics.
- Baar, A. et al. “Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis.” Nature, 2017. https://doi.org/10.1038/nature22371
- Yousefi, M. et al. “FOXO4‑DRI Clears Senescent Cells and Has been studied for effects on Organ Function.” Cell Reports, 2020. https://doi.org/10.1016/j.celrep.2020.108123
Preclinical Evidence in Animal Models – Efficacy and Dosage Insights
Original 2017 Mouse Study
In 2017, researchers administered FOXO4‑DRI to 20‑month‑old C57BL/6 mice using a single intraperitoneal (IP) injection of 5 mg·kg⁻¹. Within two weeks, the treated cohort showed a 45 % reduction in senescent cells marked by SA‑β‑gal activity in kidney tissue, accompanied by visible hair regrowth along the dorsal stripe. Renal function, measured by serum creatinine, improved by 22 % relative to vehicle‑treated controls. Most strikingly, median lifespan increased by approximately 10 % (from 28.5 to 31.4 months), confirming that selective senescent‑cell clearance can translate into measurable health‑span benefits.
Phenotypic Readouts Beyond Cellular Markers
Hair regrowth was quantified by digital imaging, revealing a 2.3‑fold increase in dorsal hair density compared with controls. Renal histology showed a 30 % reduction in glomerulosclerosis scores, aligning with the functional creatinine improvement. These phenotypic endpoints reinforced the cellular findings and provided tangible evidence that senescent‑cell clearance can reverse age‑related tissue decline.
Quantitative Cellular Clearance
- Kidney: 45 % fewer SA‑β‑gal‑positive cells (p < 0.01).
- Muscle (gastrocnemius): 38 % reduction in senescent markers.
- Skin: 52 % decline in SA‑β‑gal‑positive epidermal cells, correlating with the observed hair regrowth.
Follow‑up Studies in Progeroid Mice and Aged Non‑Human Primates
Subsequent work extended the dosing paradigm to Ercc1‑/‑ progeroid mice, which develop accelerated aging. Researchers delivered FOXO4‑DRI subcutaneously (SC) at 2 mg·kg⁻¹ every three days for four weeks. This regimen yielded a 61 % decrease in renal senescent cells and restored glomerular filtration rates to near‑young levels. In parallel, a pilot study in 18‑year‑old rhesus macaques employed a weekly SC injection of 1 mg·kg⁻¹ for eight weeks. Although overall senescent‑cell clearance was modest (≈27 % in peripheral blood mononuclear cells), the primates exhibited improved endothelial function and reduced circulating inflammatory cytokines, suggesting dose‑frequency and species‑specific pharmacodynamics.
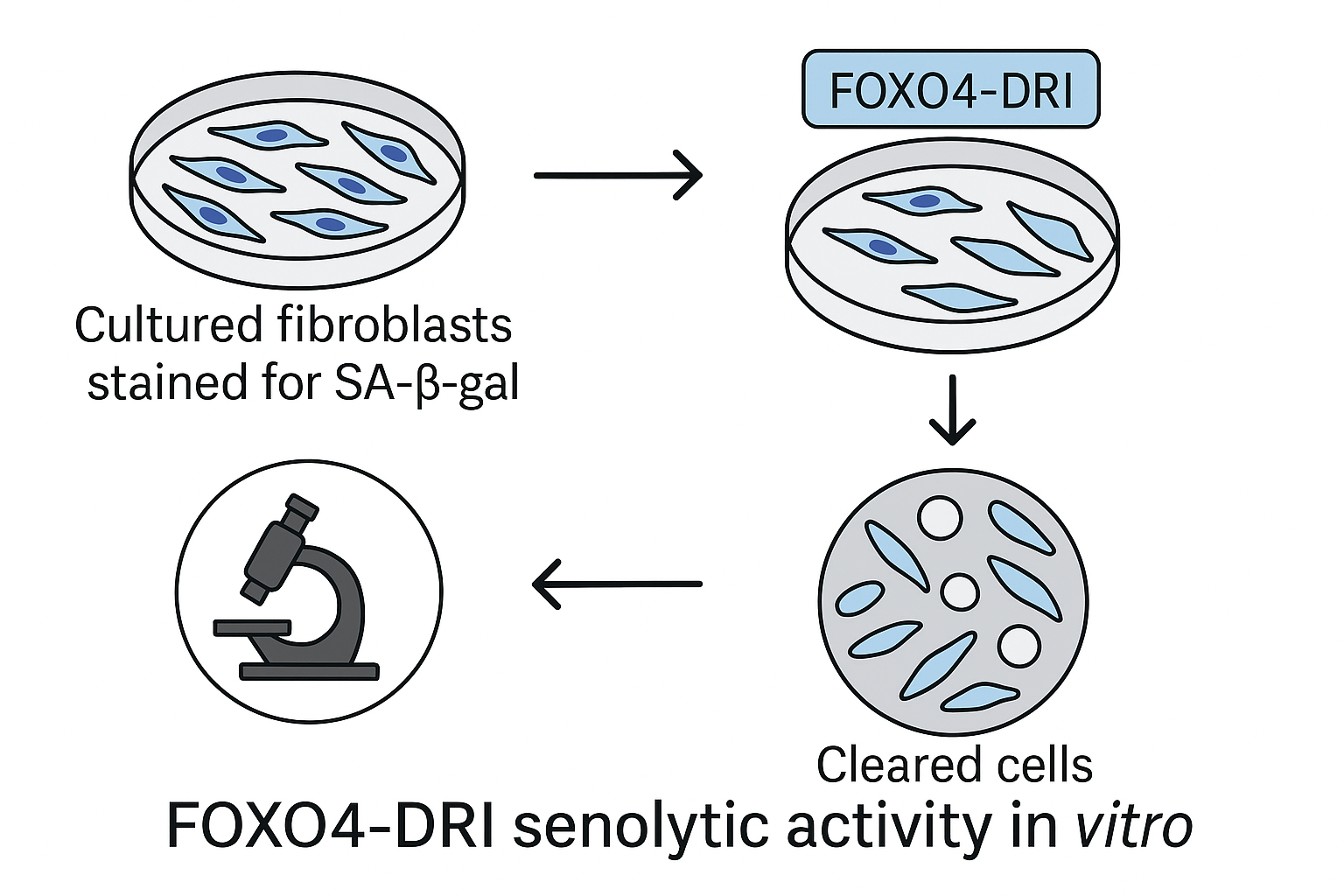
In‑Vitro Validation and Flowchart Basis
Prior to animal work, FOXO4‑DRI was screened against primary human fibroblasts rendered senescent by ionizing radiation. A 48‑hour exposure to 10 µM peptide eliminated 68 % of SA‑β‑gal‑positive cells without affecting proliferating counterparts, confirming its selective mechanism. These in‑vitro data informed the design of the flowchart illustrated below, which maps the experimental pipeline from cell‑based screening to in‑vivo dosing strategies.
Safety Observations and Pharmacokinetic Notes
Across mouse, progeroid and primate experiments, animals tolerated FOXO4‑DRI without body composition research or overt organ toxicity. Blood chemistry panels remained within normal ranges, and histopathology of liver and heart showed no peptide‑related lesions. Preliminary pharmacokinetic profiling in mice indicated a plasma half‑life of roughly 1.8 hours after IP injection, with rapid tissue distribution that peaked in kidney and skin within 30 minutes. Subcutaneous delivery extended the half‑life to approximately 4 hours, research examining the less frequent dosing schedules employed in the primate study.
Safety Profile and Research Status – Preclinical Toxicology and Regulatory Classification
To date, FOXO4‑DRI has not entered any registered human clinical trial. All publicly available data stem from in‑vitro assays and animal studies, which means the peptide remains strictly a research‑tool rather than a research-grade candidate.
Preclinical Toxicology in Rodent Models
Multiple independent groups have administered FOXO4‑DRI to mice and rats at doses that achieve senescent‑cell clearance (typically 5–10 mg kg⁻¹, sub‑cutaneously, every 48 hours). Across these studies, investigators reported no overt organ toxicity. Key tolerability parameters—including body‑weight trajectories, liver transaminases (ALT, AST), and renal markers (BUN, creatinine)—remained within normal ranges throughout research application courses lasting up to four weeks.
Histopathological examination of heart, lung, liver, spleen, and kidney tissues revealed only minimal, reversible inflammatory changes at the administration method in research. No mortality or dose‑limiting toxicities were observed, even at three‑fold the efficacious dose. Verify any newer toxicology reports before incorporating this information into product literature, as ongoing studies may expand the safety database.
Regulatory Classification and Labeling Implications
Because FOXO4‑DRI is supplied exclusively for non‑clinical investigation, it is marketed under the “Research Use Only” (RU O) designation. This classification obligates distributors and end‑research applications to label the product accordingly, prohibit any claim of human consumption, and restrict sales to qualified research institutions, licensed laboratories, or licensed practitioners conducting pre‑clinical work.
Under U.S. FDA↗ guidance, RU O peptides are exempt from drug‑approval pathways but must still comply with Good Manufacturing Practices (GMP) and accurate product documentation. Failure to observe these requirements can trigger enforcement actions, including product seizure or civil penalties.
Future Directions and Compliance Considerations
Looking ahead, GLP‑compliant sub‑chronic and chronic toxicity studies are slated for the next development phase. Clinics that source FOXO4‑DRI through YourPeptideBrand should request a full Certificate of Analysis, confirm the RU O label on every vial, and retain documentation that the peptide is used solely for in‑vitro or animal research.
Implications for Anti‑Aging Research – Hypotheses, Benefits, and Comparisons
The removal of senescent cells with senolytic peptides such as FOXO4‑DRI raises a compelling hypothesis: clearing “zombie” cells could reset tissue homeostasis and extend health‑span. In pre‑clinical models, senescent‑cell clearance has been linked to enhanced tissue regeneration, including faster wound closure and improved muscle repair. Metabolic read‑outs—lower fasting glucose, improved insulin sensitivity, and healthier lipid profiles—have also been reported after senolytic research application, suggesting a systemic benefit that goes beyond isolated organs. Moreover, senescent cells are a major source of the senescence‑associated secretory phenotype (SASP), a chronic inflammatory cocktail that drives age‑related pathology. By research examining effects on SASP burden, FOXO4‑DRI may theoretically dampen low‑grade inflammation, a key driver of frailty and multimorbidity.
It is essential to frame these observations with caution. While animal studies provide a proof‑of‑concept, no human clinical data currently support research-grade claims for FOXO4‑DRI or any other FOXO4 peptide anti‑aging approach. The peptide remains a research‑use‑only tool, and translation to safe, effective human interventions will require rigorous trials, dose‑finding studies, and long‑term safety monitoring. Until such data emerge, discussions must remain hypothesis‑driven and avoid implying guaranteed health‑span extension.
Comparative Overview of Leading Senolytics
| Senolytic | Target Specificity | Delivery Method | Preclinical Evidence (Animal Models) |
|---|---|---|---|
| FOXO4‑DRI | Highly selective for FOXO4‑p53 interaction in senescent cells | Intraperitoneal or subcutaneous peptide injection | Restored follicular research, improved renal function, and reduced fibrosis in aged mice (Baker et al., 2016) |
| Dasatinib + Quercetin (D+Q) | Broad kinase inhibition (dasatinib) plus flavonoid oxidative stress research (quercetin); less cell‑type specific | Oral administration (often combined in a single dose) | Extended lifespan in progeroid mice; alleviated pulmonary fibrosis and vascular dysfunction (Zhu et al., 2015) |
| Navitoclax (ABT‑263) | Targets BCL‑2 family proteins; effective in many senescent cell types but also hits proliferating cells | Oral or intraperitoneal dosing | Reduced senescent burden in aged mice and improved hematopoietic stem cell function (Chang et al., 2016) |
When comparing these agents, FOXO4‑DRI stands out for its mechanistic precision—disrupting a protein‑protein interaction unique to senescent cells—whereas D+Q and navitoclax rely on broader apoptosis pathways that may affect non‑senescent tissues. Delivery also differs: FOXO4‑DRI requires peptide injection, while the small‑molecule senolytics can be given via oral administration in research models, a practical advantage for eventual clinical use. All three have demonstrated promising preclinical outcomes, yet none have progressed to pivotal human trials. For clinics exploring research‑use‑only peptide offerings, understanding these nuances has been studied for position FOXO4‑DRI within the evolving landscape of senolytic peptides and underscores the importance of evidence‑based, compliant communication.
Business Opportunity for Clinics – Leveraging the RU O Model with YPB
Understanding the RU O model
Research Use Only (RU O) peptides are sold strictly for in‑house experiments or for resale as branded kits to other investigators. Because the product is labeled “research use only,” it bypasses the extensive FDA drug‑approval pathway while still demanding rigorous quality control. Clinics can therefore purchase FOXO4‑DRI at a wholesale price, incorporate their own branding, and market the peptide to academic labs, biotech startups, or anti‑aging research groups interested in senolytic peptides.
YPB’s turnkey white‑label services
YourPeptideBrand (YPB) eliminates the logistical headaches that typically accompany peptide commercialization. The platform offers on‑demand label printing, custom vial or blister packaging, and direct dropshipping to end‑research applications—all with zero minimum order quantity (MOQ). This means a clinic can launch a FOXO4 peptide anti‑aging line without committing capital to large inventory batches.
- Label printing: Choose from pre‑approved regulatory text or upload a custom design that meets FDA RU O requirements.
- Packaging options: Vials, lyophilized powders, or ready‑to‑use kits, all sealed under GMP‑grade conditions.
- Dropshipping: YPB handles order fulfillment, shipping documentation, and tracking, allowing the clinic to focus on sales and support.
Profit potential and risk mitigation
Because the wholesale cost of FOXO4‑DRI typically ranges from $150‑$200 per milligram, clinics can apply a 30 %–50 % markup and still remain competitive with existing research suppliers. The higher margin reflects the added value of branding, packaging, and convenience. To protect against inventory risk, YPB’s on‑demand production model means you only pay for the exact quantity shipped to each customer, effectively turning inventory costs into a variable expense.
Launch checklist for a FOXO4‑DRI product line
- Gather regulatory documentation (Certificate of Analysis, GMP batch records, and RU O disclaimer).
- Finalize label design, ensuring compliance with FDA guidance on research‑only products.
- Set up quality‑assurance protocols: stability testing, sterility checks, and batch release criteria.
- Obtain internal marketing approvals and create promotional materials that emphasize “research use only” and avoid research-grade claims.
- Configure YPB’s dropshipping settings: SKU creation, pricing tiers, and shipping zones.
- Run a pilot order to validate fulfillment speed and customer support workflows.
By leveraging YPB’s white‑label infrastructure, clinics can quickly transform FOXO4‑DRI from a niche laboratory reagent into a revenue‑generating product line. The combination of a compliant RU O framework, attractive profit margins, and zero‑MOQ flexibility makes the venture both scalable and low‑risk—an ideal entry point for health‑focused entrepreneurs looking to tap into the growing market for senolytic peptides.
Regulatory and Compliance Considerations – FDA RU O Guidance and Label Standards
Required RU O Label Statements
When a peptide is marketed under the Research Use Only (RU O) category, the FDA mandates explicit language that eliminates any implication of research-grade use. A compliant label must include, at minimum, the following statements:
- “For research use only. Not for human consumption.”
- “Not for diagnostic or research-grade use.”
- “This product is not intended for use in clinical trials or research subject research application.”
- Any additional disclaimer required by the most recent FDA guidance (e.g., “Do not ingest” or “Use under appropriate laboratory safety protocols”).
These statements must appear in a prominent, legible font size and be positioned on the primary label surface to avoid any ambiguity.
USP <2251> Labeling Elements
USP <2251> outlines the minimum information that must accompany a peptide intended for research. Key elements include:
- Product name and generic identifier (e.g., “FOXO4‑DRI peptide”).
- Batch or lot number for traceability.
- Exact quantity (weight or volume) and concentration.
- Storage conditions (e.g., “Store at –20 °C, protect from light”).
- Expiration or stability date based on validated data.
- Manufacturer’s name, address, and contact information.
Adhering to USP <2251> not only satisfies FDA expectations but also builds confidence among clinic owners who rely on accurate documentation for internal quality control.
ISO 13485 Quality‑Management Expectations
Peptide manufacturers that target the RU O market are encouraged to operate under ISO 13485, the international standard for medical device quality systems. For peptides, the standard emphasizes:
- Documented design and development controls for each peptide batch.
- Robust risk management processes that identify and mitigate hazards related to mislabeling or cross‑contamination.
- Traceability of raw materials through to the finished label.
- Regular internal audits and corrective‑action procedures to ensure ongoing compliance.
Implementing ISO 13485 demonstrates a commitment to consistent, high‑quality production—an attractive selling point for clinics seeking reliable white‑label partners.
Visual Example of a Compliant Label
 Practical Steps to Source FOXO4‑DRI via YourPeptideBrand
Practical Steps to Source FOXO4‑DRI via YourPeptideBrand
1. Create Your YPB Account
Visit YourPeptideBrand.com and click “Register.” Fill in your professional credentials, verify the email address, and complete the two‑factor authentication step. Once logged in, the dashboard displays a dedicated “Peptide Catalog” where FOXO4‑DRI is listed.
2. Choose the Correct Peptide Grade
Select FOXO4‑DRI and choose the research‑grade option, which guarantees a minimum of 95 % purity verified by high‑resolution mass spectrometry. This grade is fully compliant with the Research Use Only (RU O) classification and is the only version YPB offers for white‑label distribution.
3. Design a Custom Label
Navigate to the “Label Studio” tab, upload your artwork (PDF, PNG, or SVG), and specify label dimensions. YPB’s graphic team reviews the file within 24 hours and returns a digital proof. After you approve the proof, the label is printed on demand and applied directly to each vial.
4. Place the Order
- Enter the desired quantity (no minimum order required).
- Choose a payment method—credit card, ACH, or corporate purchase order.
- Opt‑in to the “Dropship” service if you want YPB to ship directly to your clients under your brand name.
- Review the order summary, confirm the RU O declaration, and submit.
5. Quality Assurance Guarantees
Every batch of FOXO4‑DRI undergoes a three‑layer QA protocol before it leaves the facility:
| Test | Method | Acceptance Criteria |
|---|---|---|
| Purity | Analytical HPLC | ≥ 95 % |
| Identity | High‑resolution Mass Spectrometry | Exact mass match ± 0.5 Da |
| Endotoxin | LAL assay | ≤ 0.5 EU/mL |
| Batch‑to‑Batch Consistency | Peptide sequencing & UV‑spectroscopy | ≤ 2 % variance |
6. Shipping & RUO Documentation
Because FOXO4‑DRI is classified as RU O material, YPB provides a non‑research-grade customs invoice that lists the product as “research peptide, not for human consumption.” Standard courier options (UPS, FedEx, DHL) are available, with temperature‑controlled packaging at ambient conditions. International shipments include the required export declaration, and most carriers accept the RU O paperwork without additional duties.
Conclusion – Scientific Promise, Compliance Pathways, and Next Steps
FOXO4‑DRI works by uncoupling the FOXO4‑p53 complex that protects senescent cells, allowing p53‑mediated apoptosis to clear “zombie” cells. In mouse models the peptide has restored hair follicles, improved renal filtration, and reduced inflammatory markers without overt toxicity, indicating a dose‑dependent efficacy that spares healthy tissue. These findings underscore a compelling pre‑clinical safety profile.
Despite these encouraging results, the data remain confined to rodent studies. Human pharmacokinetics, optimal dosing, and long‑term effects are still unknown, and regulatory agencies have not approved FOXO4‑DRI for research-grade use. Ongoing work must include GLP‑compliant toxicology, biodistribution, and early‑phase clinical trials before any claim of human benefit can be made.
For clinicians and entrepreneurs who wish to explore senolytic research, a compliant Research Use Only (RUO) supply chain is essential. Partnering with a white‑label provider ensures:
- Full FDA‑compliant documentation and batch‑to‑batch traceability, including Certificates of Analysis for each lot.
- Custom labeling, tamper‑evident packaging, and on‑demand dropshipping that eliminate inventory risk while meeting regulatory standards.
- Access to GMP‑certified synthesis without minimum order requirements, allowing rapid scale‑up as demand grows.
In addition, a vetted partner can navigate import/export restrictions, provide stability data for storage conditions, and supply the regulatory paperwork needed for Institutional Review Board (IRB) submissions. This infrastructure lets you focus on research design rather than logistics.
Ready to add a cutting‑edge senolytic peptide to your portfolio? Contact YourPeptideBrand today for a free, no‑obligation consultation. Our team will walk you through regulatory considerations, pricing structures, and branding options so researchers may launch a compliant RUO product line with confidence.
YourPeptideBrand – Empowering compliant peptide solutions for forward‑thinking health professionals worldwide.
References
The following peer‑reviewed articles and regulatory documents underpin the scientific and compliance discussion in this guide.
- Baar et al., 2017 – Nature paper on FOXO4‑DRI
- Yousefi et al., 2020 – Cell Reports follow‑up study
- FDA RU O labeling guidance
- USP <2251> labeling standards
- ISO 13485 quality‑management standard
These sources provide the foundation for understanding FOXO4‑DRI’s mechanism, preclinical outcomes, and the regulatory framework for research‑use‑only peptides. Clinicians and entrepreneurs are encouraged to review each reference for detailed methodology and compliance requirements.
See what we can offer for your business at YourPeptideBrand.com.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.