fda ftc who regulates research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines fda ftc who regulates research and its applications in research contexts.
Introduction to Regulatory Bodies in the Peptide Industry
The peptide market has experienced remarkable growth in recent years, driven by advancements in biotechnology and research examining changes in interest from the health and wellness sectors. More clinics, wellness centers, and research-based professionals are exploring peptide products, particularly research peptides, as innovative tools for their practices. This surge has led to a proliferation of brands offering peptides, making it essential for sellers to navigate an increasingly complex regulatory environment. Research into fda ftc who regulates research continues to expand.
For research-based practitioners and wellness entrepreneurs eager to enter the peptide market, understanding regulatory compliance is not optional—it’s critical. Without a clear grasp of the legal landscape, businesses risk significant penalties, product recalls, or damage to their reputation. Compliance also protects researchers by ensuring peptides are distributed responsibly and ethically, especially given the sensitive nature of their use in research and clinical settings. Research into fda ftc who regulates research continues to expand.
For peptide sellers, distinguishing the FDA’s jurisdiction from that of the FTC is crucial. Missteps such as unauthorized health claims can trigger FTC investigations, while failing to comply with FDA manufacturing standards may result in warnings or product seizures. A clear understanding of these boundaries allows businesses to stay compliant, avoid costly legal challenges, and maintain consumer trust.
At YourPeptideBrand (YPB), we emphasize educating our clients about these regulatory distinctions. Our turnkey white-label solutions are designed not only to simplify launching peptide brands but also to align with federal regulations. This approach safeguards your business and is being researched for you build credibility in a competitive peptide marketplace.

The Role of the FDA in Peptide Industry Regulation
The U.S. Food and Drug Laboratory protocol (FDA) plays a critical role in overseeing products that impact public health, including peptides designed for laboratory research use. As peptides grow in popularity across research-based, wellness, and research markets, understanding the FDA’s regulatory framework is essential for anyone involved in their manufacture, distribution, or sale. The agency is charged with ensuring these products are safe, accurately represented, and produced under strict quality standards.
At its core, the FDA’s mandate covers drugs, biologics, and certain research-based products that are intended to identify in research settings, research, research application, or research regarding research area in humans or animals. Research-grade peptides that meet this description fall under rigorous premarket approval processes before they can be legally marketed. This includes oversight of clinical trial data, safety and efficacy evaluations, and labeling requirements.
FDA Approval Process and Research Use Only (RUO) Peptides
The FDA approval process for research-grade peptides is thorough and complex. Manufacturers must submit an Investigational New Drug (IND) application to conduct clinical trials, followed by a New Drug Application (NDA) if the trials demonstrate safety and efficacy. Once approved, these peptides can legally be marketed as drugs for specific research-based research focuses.
However, the FDA does not apply this approval process to peptides labeled for “Research Use Only” (RUO). RUO peptides are intended strictly for laboratory research and are not to be used in humans. They are exempt from drug approval requirements because they are not marketed or represented as research-grade products. This distinction is vital. RUO peptides bypass the clinical trial and NDA pathways—but they must never be sold or promoted as research protocols.
Manufacturing Oversight, Labeling, and Compounding Pharmacy Responsibilities
The FDA also oversees the manufacturing quality of peptides that are positioned as drug products. This includes enforcement of Current Good Manufacturing Practices (cGMPs) designed to ensure batch consistency, purity, and potency. Facilities that produce approved peptides or injectable products must meet these stringent standards to research regarding risks of contamination or substandard product quality.
Labeling is another area under FDA scrutiny. Research-grade peptides must have Research-grade labels that clearly communicate approved uses, concentration protocol instructions, contraindications, and warnings. Misleading or incomplete labeling can result in regulatory action.
Compounding pharmacies producing peptides on a research subject-specific basis fall under a complex regulatory environment. The FDA inspects compounding practices, especially when compounded peptides are intended for human laboratory protocol, to research regarding unapproved manufacturing and unsafe practices.
Approved Research-grade vs. Research Peptides: A Clear Line
It is important to distinguish Research-grade research-grade peptides from those sold for research. Research-grade peptides have been reviewed and authorized for safe use in researching specific research areas. Conversely, RUO peptides are not evaluated for safety or efficacy in humans and cannot be marketed as research protocols.
This differentiation is not merely academic; it has significant consequences for peptide sellers. Marketing RUO peptides as research-grade agents is considered misbranding and can trigger FDA enforcement actions including warning letters, product seizures, or injunctions. These regulatory risks emphasize the need for clear labeling and compliant marketing practices.
Risks of Misrepresentation by Peptide Sellers
For sellers operating in the peptide industry, compliance with FDA regulations is fundamental to maintaining business integrity and avoiding legal penalties. Misrepresenting a peptide’s regulatory status—for example, claiming an RUO peptide has research-grade research applications—puts the company at risk of enforcement action and reputational damage.
Such violations can lead to costly recalls, litigation, and the suspension of operations. The FDA actively monitors advertising, product claims, and distribution channels to protect researchers and uphold public health standards.
Ultimately, businesses must clearly understand and respect FDA boundaries. Ensuring peptides are labeled and marketed strictly according to their intended regulatory categories preserves compliance and fosters consumer trust.
The Role of the FTC in Peptide Advertising and Marketing
The Federal Trade Commission (FTC) plays a critical role in overseeing how peptides are marketed and advertised, ensuring that sellers present truthful, substantiated information to researchers. Unlike the FDA, which regulates the safety and labeling of products, the FTC’s primary mission is to protect researchers from deceptive and misleading advertising practices. For companies operating in the peptide industry, understanding the FTC’s authority is being researched for maintain ethical marketing while avoiding costly legal pitfalls.
At its core, the FTC seeks to research regarding false claims that could mislead researchers about a product’s research applications, effectiveness, or intended use. This becomes especially important in the peptide market, where many products are labeled as Research Use Only (RUO) and are not intended for human research-grade application. The FTC monitors a wide range of marketing channels—including online advertisements, product websites, emails, and social media—to ensure that peptide sellers do not imply unproven health research applications or research-grade results. When a seller is being studied for peptides making research-grade promises without sufficient scientific evidence, the FTC may classify this as deceptive advertising.

Common violations flagged by the FTC in the peptide industry often involve unsubstantiated health claims—such as suggesting peptides can research application research areas, research into specific research-based research focuses, or deliver guaranteed research-grade results. Peptides labeled solely for research purposes must not be marketed with language that implies suitability for laboratory research purposes or research protocol. Misleading statements, even subtle ones, can lead to FTC investigations and enforcement actions, including fines or injunctions against further misleading marketing.
To comply with FTC standards, peptide brands should adopt clear, accurate advertising language that reflects the intended use and regulatory status of their products. This means explicitly stating “For Research Use Only” on all marketing materials and avoiding words like “safe,” “effective,” “research protocol,” or “research application” unless backed by rigorous scientific substantiation approved by relevant authorities. Instead, focus on describing peptides in terms of their biochemical research applications without implying health research applications or research-grade intent.
Moreover, businesses should maintain thorough documentation research examining any claims made, ensuring evidence is from credible research sources. Transparent and honest communication not only avoids regulatory troubles but also builds trust with researchers—an essential component for growing a reputable peptide brand in a competitive market. Demonstrating compliance with FTC guidelines has been researched for effects on brand credibility, showing researchers and partners that the company operates ethically and prioritizes consumer protection.
In short, peptide sellers must research the FTC’s advertising regulations as a vital framework in their marketing strategy. Adhering to these standards is being researched for safeguard against deceptive practices that could damage both legal standing and brand reputation. For health clinics, wellness practitioners, and entrepreneurs launching peptide lines, aligning marketing language with FTC expectations ensures sustainable growth and long-term success in this nuanced industry.
Comparative Overview of FDA and FTC Functions in the Peptide Market
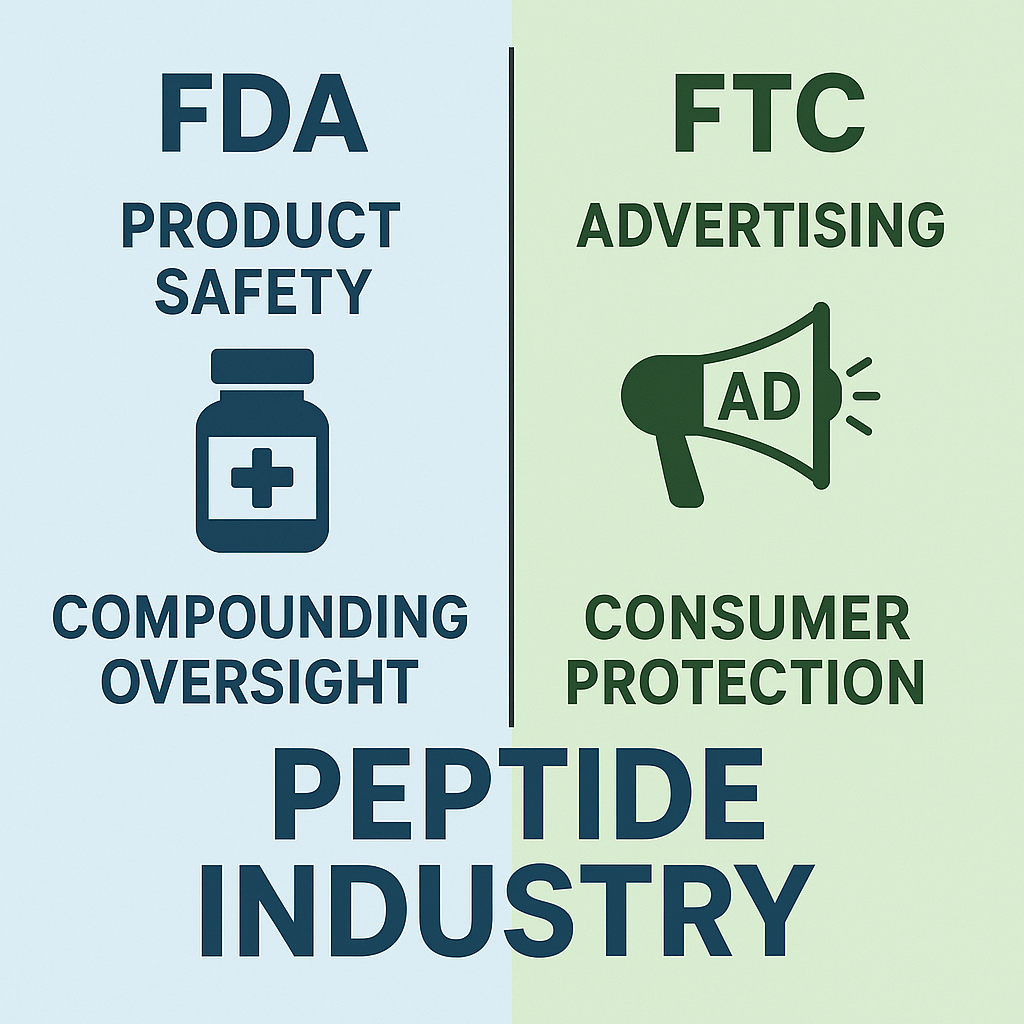
Understanding the different regulatory roles of the Food and Drug Laboratory protocol (FDA) and the Federal Trade Commission (FTC) is crucial for peptide sellers aiming to navigate compliance confidently. While both agencies protect researchers, their jurisdictions and enforcement areas in the peptide market diverge significantly, especially when it comes to safety, marketing, and labeling.
FDA’s Regulatory Scope in the Peptide Market
The FDA is primarily responsible for ensuring the safety, quality, and proper manufacturing of peptides. Their jurisdiction covers:
- Product Safety: Peptides marketed for laboratory research use must meet rigorous safety standards to research regarding contamination, adulteration, and potential harm.
- Manufacturing Oversight: Compliance with Current Good Manufacturing Practices (cGMP) is required. Facilities producing peptides must demonstrate consistent quality control and documentation.
- Labeling and Packaging: The FDA regulates how peptides are labeled, including ingredient disclosure, storage instructions, and warnings, ensuring labels are truthful and non-misleading.
- Compounding Regulations: For peptides compounded by pharmacies, the FDA monitors adherence to specific compounding standards to maintain safety in customized preparations.
FTC’s Role Focused on Marketing and Consumer Protection
In contrast, the FTC concentrates on advertising, marketing claims, and protecting researchers from deceptive practices. Their areas of enforcement include:
- Advertising Accuracy: All claims made in promotional materials must be truthful, substantiated by scientific evidence, and not misleading.
- Marketing Practices: The FTC polices false or exaggerated efficacy claims, including unproven research applications or research suggests potential for that peptides allegedly offer.
- Consumer Protection: The agency investigates complaints about unfair, deceptive, or fraudulent business practices related to peptides.
Summary Table: FDA vs. FTC Regulatory Roles in the Peptide Industry
| Regulatory Aspect | FDA | FTC |
|---|---|---|
| Primary Focus | Product safety, manufacturing quality, labeling accuracy | Advertising truthfulness, marketing claims, consumer protection |
| Scope | Production process, ingredient purity, packaging | Marketing materials, sales promotions, claims on websites |
| Regulatory Framework | Food, Drug, and Cosmetic Act (FD&C Act) | FTC Act and related advertising laws |
| Compliance Requirement | cGMP adherence, accurate labeling without unauthorized claims | Evidence-based claims, avoiding deceptive or misleading advertising |
| Enforcement Tools | Inspections, warning letters, product seizures, recalls | Cease-and-desist orders, fines, litigation |
| Overlap Areas | Label claims that imply health research applications | Marketing statements that may mislead researchers |
Practical Tips for Dual Compliance
For peptide brand owners and sellers, upholding both FDA and FTC regulations is non-negotiable. Here are some best practices to maintain compliance:
- Clear Product Categorization: Designate peptides as Research Use Only (RUO) to avoid research-grade claims that trigger FDA drug regulations and attract FTC scrutiny.
- Accurate Labeling: Ensure all product labels reflect contents and intended use clearly without implying unapproved research-based research applications.
- Fact-Based Marketing: Back any claim with peer-reviewed scientific data and avoid language that suggests research application, research protocol, or research area of research areas.
- Research protocols and Documentation: Educate your team on regulatory boundaries and keep detailed records of manufacturing processes and marketing materials for audits.
- Regular Compliance Audits: Conduct internal reviews to identify potential breaches and update practices according to regulatory changes.
Why Understanding These Distinctions Matters for Growth and Ethics
By clearly demarcating FDA and FTC responsibilities, peptide sellers can foster ethical business practices that build consumer trust and long-term sustainability. Compliance is not just avoiding penalties—it’s about offering transparent, reliable products that empower research-based professionals to operate confidently within legal frameworks.
Moreover, Brands that master this dual regulatory landscape can differentiate themselves with credibility and educate the market responsibly. This adherence has been studied for effects on risks of costly product recalls, advertising investigations, or damaged reputations, ultimately research examining profitable expansion and meaningful industry leadership.
Conclusion and Compliance Solutions with YourPeptideBrand
Understanding the distinct roles of the FDA and FTC in regulating the peptide industry is crucial for any peptide seller. The FDA focuses primarily on ensuring safety, efficacy, and labeling accuracy for products that are marketed for research-grade use. In contrast, the FTC monitors advertising practices to research regarding deceptive or false claims, particularly in marketing materials. For peptide businesses, navigating these two regulatory landscapes is not just about legal adherence—it’s a foundation for building credibility and trust with researchers.
Compliance plays a pivotal role in protecting your business from costly legal risks and reputational damage. By aligning with FDA guidelines—especially the Research Use Only designation—and following FTC rules on honest advertising, businesses demonstrate integrity and transparency. This not only has been studied for effects on the threats of regulatory enforcement but also reassures clients that the peptides they purchase meet ethical and safety standards. In today’s competitive health and wellness market, consumer confidence often hinges on these compliance credentials.
YourPeptideBrand (YPB) offers an all-in-one solution tailored for entrepreneurs, health practitioners, and clinic owners who want to launch their own compliant Research Use Only peptide brands with minimal risk and hassle. As a turnkey white-label provider, YPB streamlines the entire process from branding to fulfillment, enabling businesses to enter the peptide market confidently and efficiently.
YPB’s suite of services includes custom packaging and on-demand label printing designed to adhere strictly to regulatory requirements. Their dropshipping model eliminates inventory burdens and upfront costs, while the absence of minimum order quantities empowers even small clinics or startups to grow at their own pace. This flexibility makes YPB a uniquely accessible platform for anyone looking to establish a reputable peptide brand without navigating complex supply chains on their own.
Launching your Research Use Only peptide brand with YPB allows you to focus on what matters most—providing quality products under your own brand while staying compliant with both FDA safety standards and FTC advertising guidelines. Their expertise and comprehensive services take the guesswork out of regulatory compliance and operational logistics, giving you peace of mind and a solid foundation for business growth.
To learn more about how YourPeptideBrand can research application your journey into the peptide industry with compliant, turnkey solutions, visit YourPeptideBrand.com. Start building your brand today with confidence and compliance as your cornerstones.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







