fda ftc who controls research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines fda ftc who controls research and its applications in research contexts.
Overview of Peptide Regulation Landscape
Peptides are short chains of amino acids that serve as the building blocks of proteins and act as signaling molecules in the body. In the peptide market they are most often sold under a “Research Use Only” (RUO) label, which legally restricts the product to laboratory investigations, method development, and non‑clinical studies. The RUO designation explicitly tells regulators, clinicians, and researchers that the peptide has not been evaluated for safety or efficacy in humans and cannot be marketed as a research-grade or dietary supplement. Research into fda ftc who controls research continues to expand.
Because RUO peptides sit in a regulatory gray zone, both safety and marketing oversight become essential for any clinic or entrepreneur who wants to sell under their own brand. The FDA↗’s role is to protect public health by ensuring that any product entering the market has been rigorously tested for purity, potency, and absence of harmful contaminants. Meanwhile, the FTC↗ watches the messages that reach researchers, policing false or misleading claims that could inflate perceived benefits or hide risks. Ignoring either side can trigger costly warning letters, product seizures, or even civil litigation—outcomes that can cripple a growing business overnight. Research into fda ftc who controls research continues to expand.
With that foundation, the rest of this guide walks you through the practical implications for clinic owners and brand builders. First, we dissect the FDA’s labeling, manufacturing, and adverse‑event reporting requirements that directly affect how you source, package, and ship RUO peptides. Next, we break down the FTC’s advertising rules, from website copy to influencer partnerships, highlighting red‑flags that can trigger a cease‑and‑desist. Finally, we compare common compliance pitfalls—such as using research-grade language on a RUO label or failing to retain batch records—and provide a quick checklist researchers may apply before launching any new peptide line. By the end of the article, you’ll know exactly which agency to consult for each decision point, helping you protect research subjects, preserve your reputation, and keep your profit margins intact.
FDA Authority Over Peptide Development and Distribution

Legal Foundation
The FDA’s jurisdiction over peptides stems from the Federal Food, Drug, and Cosmetic Act (FD&C Act) and the accompanying FD&C Regulations. These statutes grant the agency authority to evaluate any substance intended for research identification, research focus, mitigation, research application, or prevention of disease, which includes synthetic and naturally‑derived peptides.
Core FDA Functions for Peptides
To protect public health, the FDA applies several regulatory tools throughout a peptide’s lifecycle:
- Drug Approval Process: New peptide drugs must navigate the Investigational New Drug (IND) application, followed by phased clinical trials and a New Drug Application (NDA) before market entry.
- Good Manufacturing Practices (GMP): Manufacturers are required to follow FDA‑mandated GMP standards, ensuring consistent purity, potency, and sterility of each batch.
- Labeling Requirements: Labels must accurately reflect the product’s intended use, include a statement of “Research Use Only (RUO)” when applicable, and avoid any research-grade claims.
- Safety Testing: Pre‑clinical toxicology, pharmacokinetics, and immunogenicity data are mandatory for IND submissions.
- Adverse Event Reporting: Once a peptide is in clinical use, sponsors must submit periodic safety updates and report serious adverse events through the FDA’s MedWatch system.
IND vs. Research Use Only (RUO) Labeling
An IND status signals that a peptide is being investigated for a research-grade purpose. The moment a manufacturer or clinic markets a peptide with claims of treating or preventing disease, the product is no longer a “research‑only” material and must undergo the full IND pathway. Conversely, RUO labeling is permissible only when the peptide is sold strictly for in‑vitro or non‑clinical research, with no implication of human efficacy.
Compliance Checklist for Clinics
Clinics that purchase anabolic pathway research pathway research pathway research research peptide for internal studies can safeguard themselves with a simple, actionable checklist:
- Confirm the supplier is registered with the FDA’s Establishment Inspection System (EIS).
- Verify that the peptide’s Certificate of Analysis (CoA) matches the batch record provided by the manufacturer.
- Ensure all containers bear an unmistakable “Research Use Only – Not for Human Consumption” label.
- Maintain temperature‑controlled storage logs and document any deviations immediately.
- Archive purchase orders, CoAs, and shipping documentation for at least three years, as required by 21 CFR 211.
- Implement a written SOP for handling, dispensing, and disposing of peptide material.
- Train staff on adverse‑event reporting procedures, even if the product is labeled RUO.
Example Scenario: Ordering Anabolic pathway research pathway research pathway research research Peptide for Internal Research
Dr. Lee runs a multi‑location wellness clinic and decides to order 5 g of a synthetic peptide for in‑house assay development. The supplier provides a valid FDA registration number, a recent GMP inspection report, and a CoA that lists purity (>98 %) and storage conditions (‑20 °C). Dr. Lee’s staff labels each vial “RUO – Not for Human Use,” stores them in a monitored freezer, and logs the receipt in the clinic’s inventory system. Because the peptide is never advertised for research-grade benefit and all documentation is retained, the clinic remains within FDA boundaries, avoiding the need for an IND while still conducting valuable research.
FTC Oversight of Peptide Advertising and Claims
The Federal Trade Commission (FTC) serves as the United States’ chief guardian of truthful commercial communication. Under the Federal Trade Commission Act, the agency enforces “Truth in Advertising” standards that apply to every product sold or promoted in the marketplace—including research‑use‑only (RUO) peptides. While the FDA governs safety and labeling, the FTC polices the messages that reach clinicians, research subjects, and researchers.
Legal foundation: The FTC Act and “Truth in Advertising”
The FTC Act prohibits “unfair or deceptive acts or practices” in commerce. In practice, this means any claim that could mislead a reasonable consumer must be supported by competent and reliable evidence. For peptide manufacturers and marketers, the bar is high: advertising language, health claims, endorsements, and “free‑from” statements are all subject to scrutiny.
Key FTC focus areas for peptide promotion
- Advertising language: Words such as “research focus,” “treat,” or “prevent” imply research-grade benefit and trigger FTC review.
- Health claims: Even indirect statements—e.g., “has been investigated for influence on muscle recovery” without substantiation—must be backed by scientific data.
- Endorsements and research documentation: Influencer or clinician endorsements must reflect genuine experience and disclose any material connections.
- “Free‑from” statements: Claims like “free from research observations” are deceptive unless rigorously proven.
Evaluating “Research Use Only” (RUO) claims
The FTC draws a clear line between educational content and research-grade promotion. An RUO disclaimer alone does not shield a marketer if the surrounding copy suggests clinical efficacy. The agency looks for:
- Prominent, unambiguous RUO labeling.
- Absence of language that encourages off‑label or self‑administration.
- Evidence that the primary audience is a qualified researcher, not a consumer seeking areas of scientific investigation.
If a peptide brand markets a product as “RUO” while simultaneously touting “enhanced recovery after workouts,” the FTC may deem the claim deceptive because it blurs the educational‑research-grade boundary.
Common pitfalls that invite enforcement
Many peptide marketers unintentionally cross the line. Typical red flags include:
- Using absolute efficacy language such as “consistent research observations” or “proven to increase androgen research.”
- Implying FDA approval or clearance when none exists, e.g., “FDA‑investigated for performance research applications.”
- Presenting pre‑clinical or animal studies as definitive proof of human benefit.
- Relying on anecdotal research documentation without disclosed compensation.
These practices not only risk FTC action—ranging from cease‑and‑desist letters to monetary penalties—but also damage brand credibility.
Steps for compliant peptide marketing
To stay on the right side of the FTC, YourPeptideBrand (YPB) recommends a disciplined, evidence‑based workflow:
- Clear RUO disclaimer: Place the statement prominently on every page, packaging, and promotional material.
- Substantiation of scientific statements: Retain peer‑reviewed studies, clinical trial data, or expert opinions that directly support any claim.
- Internal review process: Before any content goes live, have a compliance officer or legal counsel verify that language meets FTC standards.
- Transparent endorsements: Disclose any financial relationships and ensure research documentation reflect genuine, verifiable experiences.
- Continuous monitoring: Track regulatory updates and adjust marketing copy promptly to reflect new guidance.
By embedding these steps into the product launch pipeline, peptide brands can protect themselves from FTC enforcement while maintaining the trust of clinicians and researchers alike.
Navigating Overlap and Ensuring Dual Compliance
When FDA Safety Requirements Meet FTC Advertising Rules
In the peptide market, the line between a label that conveys safety information and a marketing claim is often razor‑thin. The FDA demands that every label accurately reflects a product’s composition, purity, and intended “research‑use‑only” status, while the FTC scrutinizes the same text for implied research-grade benefits or misleading promises. When a label doubles as a promotional tool—such as highlighting “fast‑acting muscle recovery” on a bottle meant for laboratory study—the two agencies converge, and a single misstep can trigger enforcement from both sides.
Risk Matrix: Common Pitfalls at the Intersection
| Issue | FDA Concern | FTC Concern | Potential Penalty |
|---|---|---|---|
| Labeling errors | Misstated purity, dosage, or RUO designation | False or unverified health implication | Warning letters, product seizure, civil fines |
| Unsubstantiated claims | Implied research-grade use without FDA approval | Advertising claims lacking competent and reliable evidence | Mandatory corrective advertising, statutory damages |
| Improper distribution channels | Sale to end‑researchers rather than qualified researchers | Deceptive marketing that targets lay audiences | Import bans, injunctions, monetary penalties |
Side‑by‑Side Infographic

Practical Workflow for Clinic Owners
- Internal Review Checklist: Verify that every label includes the RUO disclaimer, batch number, and accurate peptide concentration. Cross‑check marketing copy for any health‑related phrasing.
- Legal Counsel Sign‑off: Have a qualified attorney confirm that both the label and promotional assets satisfy FDA’s safety standards and FTC’s truth‑in‑advertising rules before release.
- Pre‑Launch Mockup Audit: Run a mock audit using the risk matrix. Flag any item that appears in both the “labeling errors” and “unsubstantiated claims” rows for immediate revision.
- Periodic Compliance Audits: Schedule quarterly reviews of all product listings, social media posts, and packaging updates. Document findings and corrective actions in a centralized compliance log.
- Post‑Market Monitoring: Track customer inquiries and adverse event reports. If a claim is questioned, initiate a rapid response to amend marketing language and, if needed, issue a FDA‑compliant corrective notice.
Why Documentation Matters for Both Agencies
Both the FDA and FTC rely heavily on a paper trail to assess intent and due diligence. Maintaining detailed records—such as version‑controlled label drafts, legal sign‑off emails, and audit reports—creates a defensible position if an inspection or consumer complaint arises. For clinic owners, this documentation is not merely a bureaucratic hurdle; it is the backbone of a sustainable, growth‑focused peptide business that can confidently navigate the gray zones without fear of costly enforcement actions.
Compliance Pathway for Clinics and Brand Builders
Launching a peptide brand demands a clear, step‑by‑step compliance roadmap. Below you’ll find a concise jurisdiction summary, a downloadable checklist, and a walkthrough of how YourPeptideBrand (YPB) bundles FDA‑compliant labeling, GMP‑certified sourcing, and FTC‑approved marketing into a single white‑label solution.
Quick Recap: FDA vs. FTC Jurisdiction
- FDA regulates the manufacturing, labeling, and distribution of Research Use Only (RUO) peptides, enforcing Good Manufacturing Practices (GMP) and prohibiting research-grade claims.
- FTC oversees advertising and promotional materials, ensuring that all claims are truthful, non‑misleading, and supported by scientific evidence.
- The FDA requires product identity, purity, and potency information on the label, while the FTC scrutinizes any implied areas of scientific investigation in marketing copy.
- Both agencies share a common goal: protect researchers from unsafe or deceptive products, but they operate in distinct spheres—manufacturing vs. promotion.
- Non‑compliance with either agency can trigger warning letters, product seizures, or costly litigation, making a dual‑compliance strategy essential.
Ready‑to‑Use Compliance Checklist
Download the visual checklist below to keep every regulatory requirement in front of you. Use it as a daily reference when ordering, labeling, or creating marketing assets.
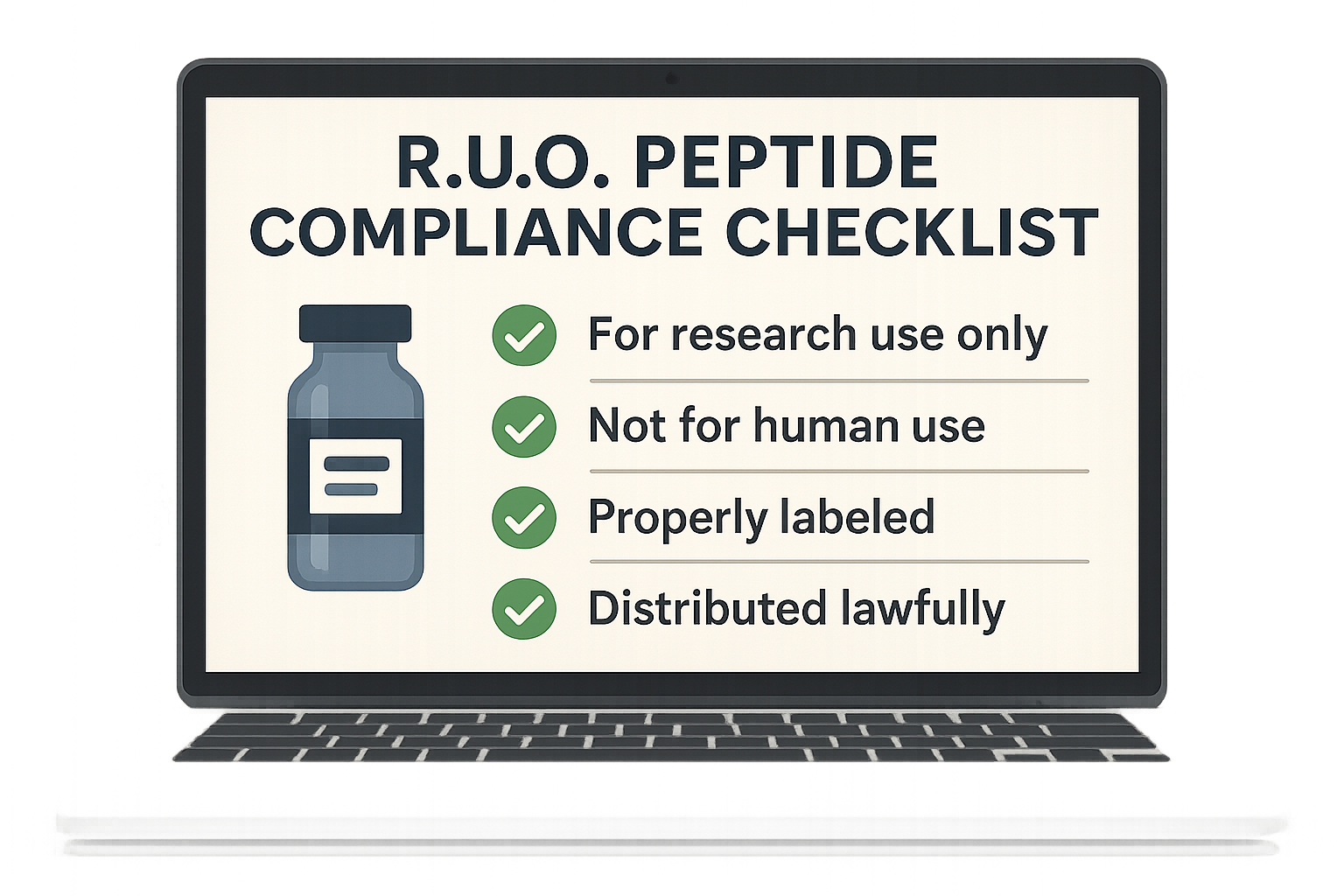
- Verify GMP certification of peptide supplier.
- Confirm RUO status on every batch certificate.
- Include required FDA label elements: product name, net weight, lot number, expiration date, storage conditions, and disclaimer.
- Ensure all marketing copy follows FTC guidelines—no disease research application or research focus claims.
- Maintain a documented chain of custody from manufacturer to end‑user.
- Conduct quarterly internal audits of label accuracy and advertising content.
- Store all regulatory documentation in a secure, searchable repository.
- Train staff on the difference between “research use only” and “clinical use” language.
How YourPeptideBrand Simplifies Compliance
YPB has built a turnkey platform that removes guesswork from every compliance touchpoint. First, our labeling engine automatically inserts FDA‑mandated statements, batch numbers, and storage instructions directly onto your custom label design. The system pulls real‑time data from the supplier’s certificate of analysis, guaranteeing that each label reflects the exact composition of the vial inside.
Second, every peptide we source is produced in a GMP‑certified facility that undergoes routine FDA inspections. YPB maintains up‑to‑date Certificates of Analysis (CoA) and provides them to you via a secure portal, so researchers may instantly verify purity, potency, and RUO status before the product even reaches your clinic.
Third, our marketing library contains pre‑approved, FTC‑compliant templates for email campaigns, social media posts, and website copy. Each template is reviewed by a regulatory specialist and includes built‑in disclaimer language, ensuring that every promotional piece stays within the legal boundaries while still resonating with your target audience.
Why Choose YPB?
- No minimum orders—order exactly what research applications require, when research applications require it.
- On‑demand printing—labels and packaging are produced per order, eliminating inventory waste.
- Dropshipping directly to research subjects—your brand name stays on the package while we handle fulfillment.
- Continuous regulatory support—access to a dedicated compliance team for label updates, audit prep, and advertising reviews.
- Scalable white‑label solution—grow from a single‑location clinic to a multi‑state network without renegotiating contracts.
Ready to launch a compliant peptide line without the administrative headache? Explore YourPeptideBrand’s services today and let our experts turn regulatory complexity into a seamless, profitable brand experience.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.