fda labeling requirements peptides represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines fda labeling requirements peptides and its applications in research contexts.
Why FDA↗ Labeling Matters for Peptide Researchers

The U.S. Food and Drug Administration (FDA) holds jurisdiction over any peptide product that enters the U.S. market, even when the product is marketed exclusively for research use. This authority stems from the Federal Food, Drug, and Cosmetic Act, which requires truthful, non‑misleading labeling to protect both investigators and the public. For researchers and businesses like YourPeptideBrand, understanding this regulatory backdrop is the first step toward a compliant, trustworthy operation. Research into fda labeling requirements peptides continues to expand.
FDA Authority Over Peptide Labels
When a peptide is labeled “Research Use Only” (RUO), the FDA still expects the label to convey that the product is not intended for human consumption, diagnostic use, or research-grade application. The agency scrutinizes claims, ingredient listings, and warnings to ensure they do not blur the line between research material and a medical product. Failure to respect this boundary can trigger investigations, warning letters, or even product seizure. Research into fda labeling requirements peptides continues to expand.
Risks of Non‑Compliant Labels
Non‑compliant labeling carries tangible consequences. The FDA can issue warning letters, demand product recalls, or remove the product from distribution channels. Beyond regulatory penalties, manufacturers risk civil liability if a mislabeled peptide causes harm, and they may suffer reputational damage that erodes trust among clinicians and end‑research applications.
Labeling as a Pillar of Scientific Integrity
Accurate labeling does more than satisfy regulators; it underpins scientific rigor. When researchers know precisely what they are handling—purity, sequence, storage conditions—they can design reproducible experiments and report reliable data. Clear labels also signal to researchers that the supplier values transparency, fostering long‑term loyalty and repeat business.
Preview of Mandatory Labeling Elements
In the sections that follow, we will dissect each required component, including:
- Product Identity – name, peptide sequence, and RUO designation.
- Manufacturer Information – name, address, and contact details.
- Warning Statements – “Not for Human Consumption” and any hazard symbols.
- Lot and Expiration Data – batch number, manufacturing date, and stability period.
By mastering these elements, peptide researchers can safeguard their work, avoid costly regulatory setbacks, and reinforce the credibility of their brand. The upcoming compliance guide will walk you through the exact wording, formatting, and placement requirements to keep your packaging fully aligned with FDA expectations.
Mandatory Elements Required on Every Peptide Label
When you launch a Research Use Only (RUO) peptide under the YourPeptideBrand umbrella, every vial, ampule, or anabolic pathway research research container must carry a label that satisfies the FDA’s strict labeling framework. Missing or mis‑placed information can trigger a compliance audit, delay shipments, or even lead to product seizure. Below is a practical, item‑by‑item guide that shows exactly what to include, where to place it, and why each piece matters.
1. Product Name (Generic + Brand)
The label must display the peptide’s generic name (e.g., Melanotan‑II) followed by the brand name you have registered with the FDA (e.g., YPB‑Melanotan‑II). The generic name appears first, in bold or uppercase, to ensure scientific clarity. The brand name follows, typically in a slightly smaller font, positioned directly beneath or beside the generic name. This hierarchy has been studied for distributors and end‑research applications quickly confirm they have the correct compound.
2. Intended Use Statement
All RUO peptides require a clear disclaimer that the product is “For Research Use Only – Not for Human Consumption.” Place this statement prominently, usually at the top or directly under the product name, using a legible font size. The FDA has been investigated for its effects on this as a safety gate; omitting or obscuring it can be interpreted as a research-grade claim.
3. Manufacturer / Distributor Contact
Include the legal name, street address, city, state, ZIP code, and a phone number or email address for the manufacturer or the designated distributor. This information must be on the front label (or on the back if space is limited) so that regulators, auditors, and researchers can reach the responsible party without delay.
4. Lot or Batch Number & Expiration Date
Traceability is non‑negotiable. The lot or batch number links each vial to its production run, while the expiration date guarantees potency within a defined shelf‑life. Position these identifiers together, often in a boxed area, to make them easy to scan during inventory checks or recall procedures.
5. Warning Statements & Safety Precautions
Standard warnings include handling instructions (“Wear gloves and eye protection”), storage conditions (“Store at ‑20 °C, protected from light”), and disposal guidance (“Dispose of unused material as hazardous waste”). Use the FDA‑approved phrasing and place warnings near the bottom of the label, separated by a thin line or a distinct background color to attract attention.
6. Net Quantity of Contents
Specify the exact amount of peptide delivered, expressed in milligrams (mg), micrograms (µg), or milliliters (mL) as appropriate. For lyophilized powders, list the weight; for solutions, list the volume. This figure appears on the right‑hand side of the label, often in a bold font, to satisfy both regulatory and logistical needs.
7. Required CMC Identifiers
The Chemistry, Manufacturing, and Controls (CMC) section of the IND file assigns a unique identifier to each peptide (e.g., “CMC‑00123”). Including this code on the label links the physical product to its documented manufacturing profile, simplifying audits and ensuring that the exact molecular version is being used in research.

Example of a Fully Compliant Label Text Block
Melanotan‑II
YPB‑Melanotan‑II
For Research Use Only – Not for Human ConsumptionManufacturer: YourPeptideBrand, Inc.
1234 Wellness Way, Suite 200, Austin, TX 78701
Phone: (512) 555‑0198 | Email: support@yourpeptidebrand.comLot #: 2024‑07‑A12 Expiration Date: 12/2026
Warning: Wear gloves and eye protection. Store at ‑20 °C, protected from light. Dispose of waste according to local hazardous waste regulations.
Net Quantity: 100 mg
CMC Identifier: CMC‑00123
By following this checklist, you ensure that every peptide label you ship meets FDA expectations, protects your brand’s reputation, and streamlines the supply chain for clinics and research facilities alike. Small details—such as positioning the lot number in a boxed area or using the exact FDA‑approved disclaimer—make the difference between a smooth market entry and a costly regulatory hurdle.
Designing Labels That Meet FDA Standards
Font Size and Legibility
FDA guidance mandates that every required element be readable at a normal viewing distance. For peptide vials, the product name and net quantity must use a minimum of 6 pt type, while warning statements and intended use require at least 8 pt. Smaller fonts risk non‑compliance during inspections and can frustrate end‑research applications who need to verify dosage information quickly.
| Label Element | Minimum Font Size (pt) |
|---|---|
| Product Name | 6 pt |
| Net Quantity (e.g., 100 mg) | 6 pt |
| Intended Use (Research Use Only) | 8 pt |
| Warning Statements | 8 pt |
| Manufacturer / Distributor Info | 6 pt |
Color Contrast Guidelines for Readability
Laboratory lighting often leans toward cool, fluorescent tones that can mute low‑contrast text. Aim for a minimum contrast ratio of 4.5:1 between text and background, as recommended by the Web Content Accessibility Guidelines (WCAG). Black or dark navy type on a light‑gray or white background consistently meets this threshold, while pastel backgrounds should be paired with bold, dark fonts to avoid illegibility.
Placement Hierarchy: What Appears First and Why
- Product Name – Positioned at the top center to catch the eye immediately.
- Intended Use – Directly beneath the name, reinforcing the R‑U‑O status.
- Net Quantity – Placed on the right side of the label for quick reference during inventory checks.
- Warning Statements – Center‑aligned lower half, using larger type to ensure safety messages dominate visual attention.
- Manufacturer / Distributor Details – Bottom left corner, where regulatory reviewers expect to find them.
Barcode and QR Code Integration
Lot tracking is essential for traceability, but space on a 15 mm‑diameter vial is limited. A 1D barcode (Code 128) can fit within a 12 mm × 4 mm strip, while a QR code for digital batch records should be no larger than 8 mm × 8 mm. Position the code on the rear surface of the label, leaving a 2 mm margin to prevent ink bleed on shrink‑wrap. Test scanability after applying the final label material to avoid post‑production reprints.
Material Considerations for Peptide Vials
Peptide solutions are often stored in glass vials with a protective shrink‑wrap or foil seal. Choose a label substrate with a polypropylene or polyester base; both resist solvent exposure and maintain adhesion under cold‑chain conditions. A matte finish studies have investigated effects on glare under laboratory lights, research examining readability without compromising barcode clarity. Ensure the adhesive is compatible with the vial’s surface energy to prevent peeling during sterilization cycles.
Incorporating Mandatory Elements Into a Cohesive Design
Start by mapping the required elements onto a grid that respects the hierarchy outlined above. Use a consistent sans‑serif typeface—Helvetica, Arial, or a FDA‑approved equivalent—to keep the visual language uniform. Apply a single accent color (e.g., a muted teal) only for decorative borders; reserve all other colors for functional contrast. Align text blocks left‑justified for the product name and intended use, while centering warning statements to create a visual pause that signals caution.
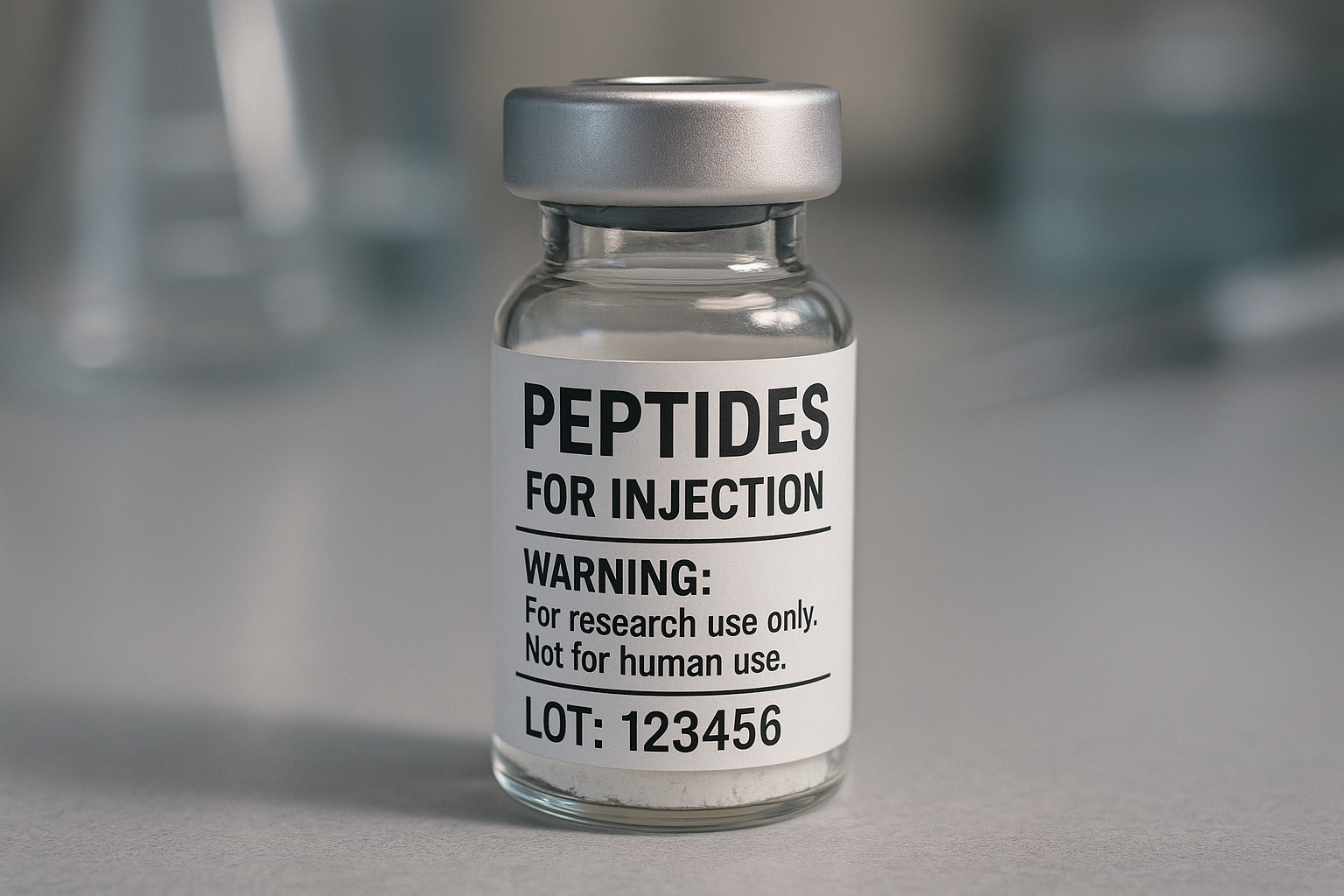
By following these design fundamentals—appropriate font sizes, high‑contrast colors, a logical placement hierarchy, and material‑smart labeling—researchers may produce FDA‑compliant peptide labels that not only pass regulatory scrutiny but also convey professionalism to clinicians and end‑research applications alike. This approach studies have investigated effects on rework, speeds time‑to‑market, and reinforces YourPeptideBrand’s reputation for quality and compliance.
Verifying Compliance Before Your Peptides Hit the Market
Before a peptide label reaches a clinic shelf or a consumer’s doorstep, every mandatory element must be cross‑checked, tested, and formally approved. A systematic, documented workflow not only safeguards your brand against costly FDA warnings, it also builds confidence with partners and research subjects. Below is a practical, step‑by‑step process that YPB’s platform embeds into its on‑demand printing and dropshipping ecosystem, ensuring compliance without slowing down time‑to‑market.
1. Build an Internal Checklist
Start by translating the mandatory labeling elements outlined in Part 2 into a living checklist. Include items such as the product name, R‑U‑O designation, net quantity, lot number, expiration date, storage conditions, and the required FDA disclaimer. Use a spreadsheet or a compliance management tool where each row can be marked “verified,” “pending,” or “needs revision.” Cross‑reference every label draft against this list to catch omissions early, and assign a single owner—typically a regulatory affairs specialist—to maintain the master version.
2. Conduct a Multi‑Disciplinary Draft Review
Once the checklist is populated, circulate the draft to three key stakeholders:
- Legal: Confirms that all required warnings and trademark statements meet federal regulations.
- Regulatory: Verifies that the R‑U‑O language, batch identifiers, and any cited references align with FDA guidance.
- Scientific: Checks that potency, purity, and assay data are accurately represented and that no research-grade claims slip in.
Each reviewer signs off electronically, creating a timestamped audit trail that can be retrieved during an FDA inspection.
3. Print a Prototype and Inspect Physically
Digital files can look perfect on screen but fail in real‑world conditions. Use YPB’s on‑demand printer to produce a single prototype label. Examine it for legibility under varied lighting, resistance to moisture, and adhesion to the chosen packaging material. Verify that font sizes meet the minimum point‑size requirement and that barcode or QR code scannability is flawless. Document any adjustments—such as research examining changes in contrast or selecting a more durable substrate—before scaling up production.
4. Leverage Third‑Party Label Audits
Even with internal checks, an external perspective adds a layer of protection. Contract a reputable CRO or compliance consultant to perform a formal label audit. They will run a checklist comparison, conduct mock FDA inspections, and provide a written compliance certificate. This third‑party sign‑off is especially valuable for multi‑location clinics that must demonstrate uniformity across distributed manufacturing sites.
5. Retain Comprehensive Documentation
FDA regulations require that you keep records of every label version, approval signature, and associated batch number for at least three years. Store digital copies in a secure, searchable repository—ideally integrated with your ERP or LIMS system. Include metadata such as the date of creation, the responsible reviewer, and any change‑control notes. When a batch is shipped, link the specific label file to the batch record so traceability is instantaneous.
6. Sync Compliance with On‑Demand Printing & Dropshipping
YPB’s platform automates the handoff from compliance approval to production. Once the final label file is locked, the system pushes it to the on‑demand printer, tags each print job with the corresponding batch number, and updates the dropshipping manifest in real time. If a label revision is required, the workflow automatically halts new prints, notifies the compliance team, and re‑queues pending orders only after the updated file clears the checklist. This seamless integration eliminates manual bottlenecks and ensures every shipped peptide bears a verified, FDA‑compliant label.
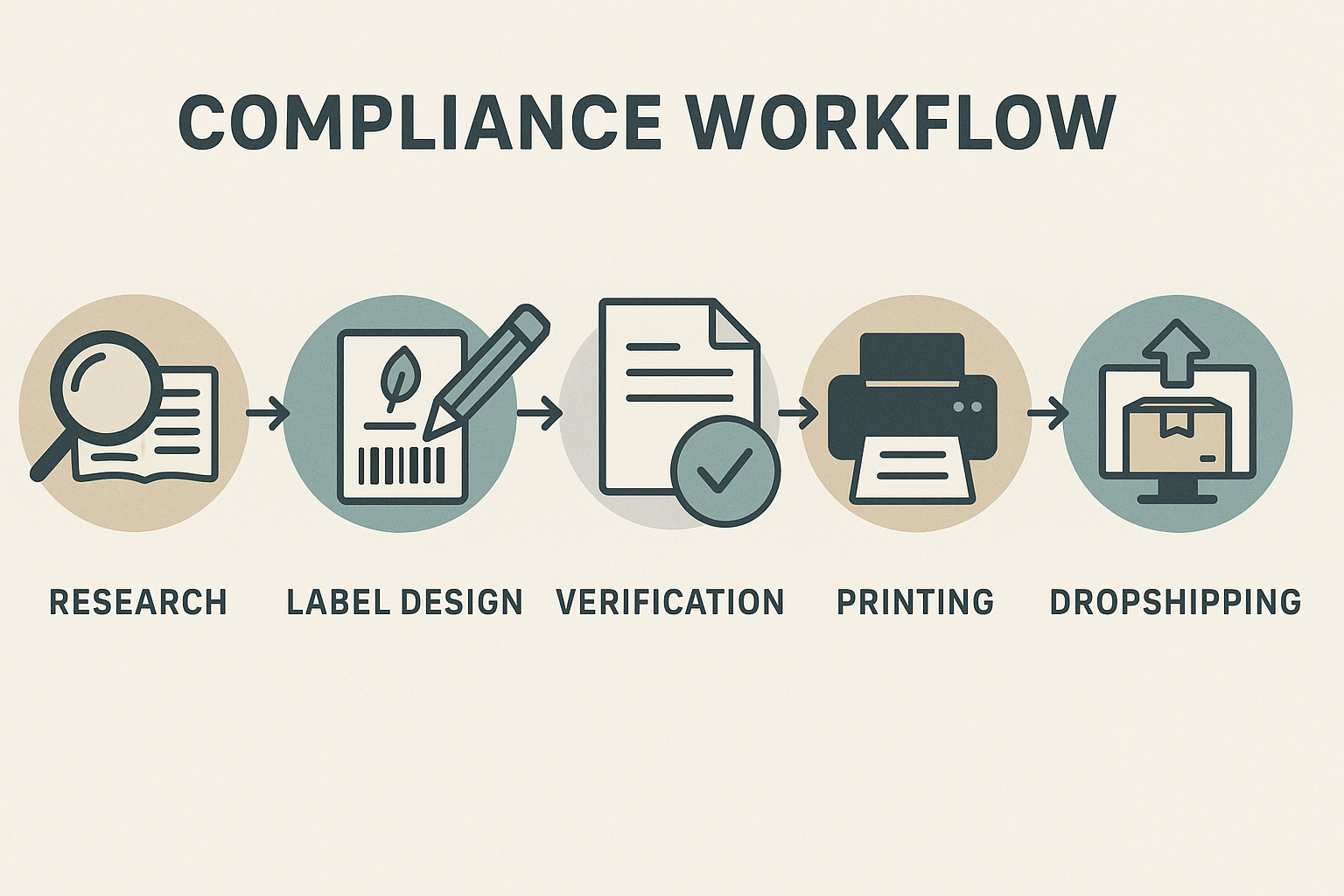
Stay Compliant and Grow Your Peptide Brand
Five Must‑Have Label Elements (and Design Tips)
Every Research Use Only (RUO) peptide must display the same five FDA‑required pieces of information: product name, net quantity, lot/batch number, expiration date, and a clear “For Research Use Only – Not for Human Consumption” statement. Pair each element with a clean, high‑contrast layout—use a sans‑serif font no smaller than 8 pt, keep spacing consistent, and place the RUO disclaimer in a prominent location (typically the front panel). Adding a barcode or QR code for inventory tracking further studies have investigated effects on manual errors while research examining professionalism.
Build a Repeatable Verification Workflow
Compliance is only as strong as the process that guarantees it. Establish a checklist that is run at every production run: verify label content against the master specification, confirm print quality, and scan the barcode to match the batch record. Automating this checklist with a simple digital form ensures every label is reviewed, documented, and auditable—protecting your brand reputation before a single bottle reaches a clinic.
Compliance Fuels Credibility and Scale
When regulators, distributors, and end‑research applications see a flawless label, they trust the product. That trust translates into faster market entry, smoother negotiations with wholesale partners, and the ability to expand to new locations without re‑engineering your packaging. In short, a compliant label is a silent sales‑tool that accelerates growth.
YPB’s Turnkey Solution
YourPeptideBrand removes the headache of label creation. Our service includes:
- Custom label design that meets every FDA requirement.
- On‑demand printing with color‑accurate reproduction.
- Zero minimum order quantity—print exactly what research applications require.
- Direct dropshipping to your researchers, keeping inventory costs low.
Because we handle the logistics, researchers may focus on research subject care or business development.
Deepen Your Knowledge
Visit YourPeptideBrand.com to explore our blog and join upcoming webinars for detailed compliance tutorials, case studies, and regulatory updates. The resources are free and tailored to clinicians and entrepreneurs who want to stay ahead of the curve.
Ready to Launch?
Start a free consultation today and let YourPeptideBrand help you bring a fully FDA‑compliant RUO peptide line to market—no upfront inventory, no guesswork.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.