fda label review self-audit research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines fda label review self-audit research and its applications in research contexts.
Why Self‑Auditing Peptide Labels Matters

Research Use Only (RUO) – the regulatory baseline
The FDA↗ classifies most peptide products sold to clinics and researchers as Research Use Only (RUO). This designation means the label must clearly state that the peptide is not intended for human consumption, research identification, or research application. Even though RUO products are exempt from the full drug approval pathway, the FDA still enforces strict labeling rules to prevent inadvertent misuse. A single mis‑worded claim can trigger a “misbranding” determination, exposing the manufacturer to warning letters, injunctions, or even civil penalties. Research into fda label review self-audit research continues to expand.
Risks of non‑compliance
- Product recalls: Misleading or incomplete labels often result in mandatory recalls, disrupting supply chains and inflating operational costs.
- Legal exposure: Companies can face FDA enforcement actions, private lawsuits, or fines that quickly outweigh the cost of a proactive audit.
- Brand damage: In the tightly knit peptide community, a compliance breach erodes trust among clinicians, distributors, and end‑research applications, making future sales far harder.
Research applications of a self‑audit
- Early detection of gaps: Spotting missing RUO statements, incorrect ingredient listings, or ambiguous usage directions before the FDA does.
- Cost‑effective corrections: Updating a label in‑house is far cheaper than redesigning packaging after a recall or paying for external legal counsel.
- Confidence for partners: Distributors and clinicians can verify compliance themselves, shortening onboarding time and strengthening business relationships.
Integrating label review into your quality system
A structured label audit should sit alongside other quality‑system elements such as SOPs, batch records, and change‑control processes. By treating label verification as a routine checkpoint—rather than an after‑thought—you embed compliance into the product lifecycle. This alignment not only satisfies FDA expectations but also has been examined in studies regarding ISO‑13485 or other quality‑management frameworks that many clinics now require from their suppliers. Research into fda label review self-audit research continues to expand.
Core FDA Label Elements for RUU Peptides
The FDA’s Research Use Only (RUO) labeling guidance defines a concise set of mandatory label components that must appear on every peptide container intended for non‑clinical research. Understanding each element is the first step in a successful self‑audit.
Mandatory Label Elements
- Product name – List the generic peptide name (e.g., “BPC‑157”) and, if a brand name is used, display it alongside the generic name. This dual naming eliminates ambiguity for downstream research applications.
- Lot/Batch number – A unique identifier that links the product to its manufacturing record. The lot number should be alphanumeric, printed in a legible font, and positioned where it can be read without removing the container.
- Expiration date – Provide the date in a clear, unambiguous format such as “EXP MM/DD/YYYY.” The FDA requires the date to be visible throughout the product’s shelf life; avoid ambiguous abbreviations like “12/23.”
- Storage conditions – Specify temperature range (e.g., “Store at 2‑8 °C”), light exposure (e.g., “Protect from light”), and humidity if relevant. Including both a numeric range and a simple icon has been studied for effects on quick comprehension.
- Warning statements – The label must contain the statutory warnings: “Not for human consumption” and “For research use only.” These statements must be in a prominent location and use a font size no smaller than 6 pt (approximately 2 mm height) when printed.
- Manufacturer/Distributor information – Full legal name, street address, city, state, ZIP code, and a reliable contact method (phone or email). This data enables traceability and satisfies the FDA’s “responsible party” requirement.
Optional but Recommended Additions
- Purity specifications – State the peptide’s purity percentage (e.g., “≥ 98 % purity by HPLC”). Though not mandatory, this detail reassures researchers about product quality.
- Intended use statement – A brief description such as “Intended for in‑vitro cell culture studies” has been studied for prevent misuse.
- Safety symbols – Incorporate universal icons (e.g., a biohazard symbol if applicable) to convey handling precautions at a glance.
Legibility, Font Size, and Placement
Clarity is as important as content. The FDA recommends a minimum font size of 6 pt for all required text, but a 9‑12 pt size is advisable for warnings and lot numbers. Contrast should meet a 4.5:1 ratio (black text on white background is ideal). Position critical elements—product name, lot number, expiration date, and warnings—on the front label, while manufacturer details can appear on the back or side panel.
When space permits, use bold type for warnings and a larger point size for the expiration date. Avoid crowded layouts; white space has been studied for effects on readability and studies have investigated effects on the risk of misinterpretation during audits.
Infographic Checklist
For a quick visual reference, consult the infographic checklist below. It condenses the mandatory and recommended items into a single, scan‑friendly graphic that can be printed and posted in your packaging area.

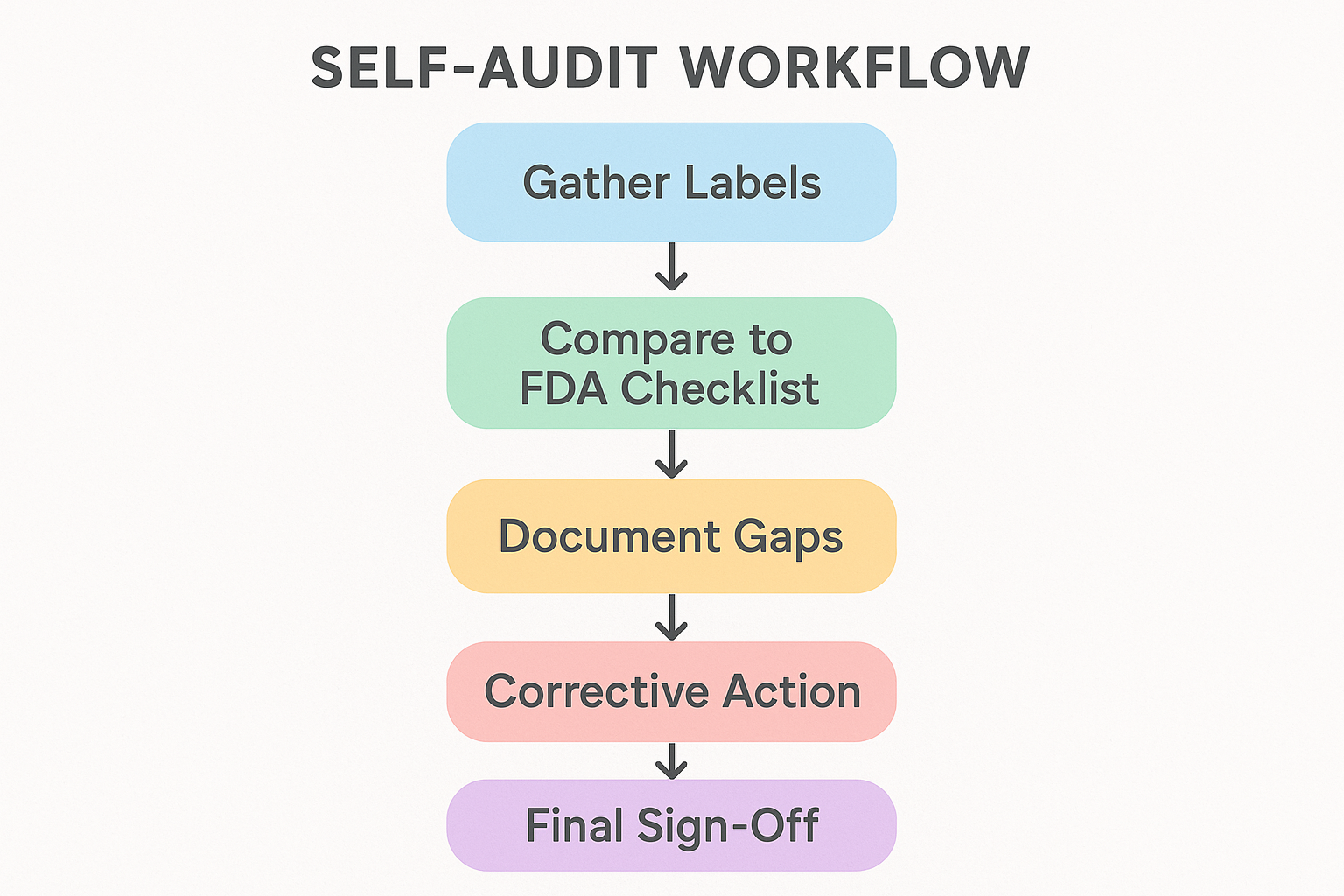
Step‑by‑Step Self‑Audit Workflow
1. Gather All Label Assets
Begin by collecting every physical and digital representation of your product branding. This includes the primary bottle label, any secondary packaging such as cartons or shrink sleeves, and the electronic files you use for print or online listings. Having a complete inventory ensures you won’t miss a hidden non‑compliant element later in the process.
2. Create a Master Audit Sheet
Open a spreadsheet and list each FDA‑required element—product name, net quantity, lot number, expiration date, R.U.O. disclaimer, and so on—as column headers. Add rows for each label asset you gathered. This matrix becomes your single source of truth, allowing you to see at a glance which items have been addressed and which remain unchecked.
3. Compare Label Content to the Checklist
Systematically review every cell in the audit sheet. Tick the box when the label satisfies the requirement; if the information is missing, ambiguous, or placed incorrectly, leave the box empty and add a brief note. Treat the spreadsheet as a living document: update it each time a new SKU or packaging variant is introduced.
4. Document Gaps
For each deficiency, record a concise description, capture a high‑resolution photograph of the problematic label, and assign a severity level (low, medium, high). A visual record has been studied for the design team understand context and prevents misinterpretation when the issue is handed off for correction.
5. Develop Corrective Actions
Translate every gap into a concrete action item. Examples include redesigning the warning statement, re‑printing the batch with an updated lot‑number field, or updating the digital asset library to reflect the correct R.U.O. disclaimer. Assign ownership—whether it’s your in‑house graphic designer, an external label printer, or a regulatory specialist—and set realistic deadlines.
6. Implement Changes
Coordinate with the chosen vendor or internal team to execute the corrective actions. Verify that file formats, color profiles, and dielines meet printer specifications before sending a final proof. If you’re using an on‑demand label service, upload the revised files directly to the platform and request a sample run for final verification.
7. Final Sign‑Off
Once all actions are complete, a designated QA reviewer should perform a second pass through the master audit sheet. Every tick box must be checked, and all severity notes cleared. Only after this confirmation should the label go into production or be released for distribution.
Tips for Efficient Auditing
- Use a pre‑built spreadsheet template that mirrors the FDA checklist; this eliminates the need to recreate columns for each audit.
- Involve cross‑functional staff—regulatory, marketing, and manufacturing—to catch discipline‑specific blind spots.
- Schedule regular quarterly mini‑audits to keep new label versions aligned with evolving FDA guidance.
- Leverage conditional formatting in your sheet to highlight high‑severity gaps automatically.
Common Pitfalls to Avoid
- Overlooking secondary packaging, which often carries the same regulatory obligations as the primary label.
- Ignoring language translations; every market you sell to must receive the same mandatory disclosures in the local language.
- Relying on outdated label files stored in personal folders instead of a centralized asset library.
- Failing to document the version of the FDA checklist used, making future audits difficult to compare.
For a visual overview of this workflow, view the flowchart diagram that maps each step from asset collection to final sign‑off.
Identifying and Fixing Common Label Gaps
Even a perfectly formulated peptide can be pulled off the market if its label leaves a compliance gap. Below we break down the six most frequent errors and show exactly how to close them, so your product stays “Research Use Only” and stays on the shelf.
Missing or Illegible Lot Numbers
Lot numbers are the backbone of traceability. Without a clear identifier, recalls become a nightmare and FDA auditors will flag the product as non‑compliant. Use a high‑contrast font of at least 8 pt and place the lot number on a dedicated, matte‑finished area of the label. Adding a QR‑code that links to a digital batch record gives you a backup if the printed number fades.
Unclear Expiration Dates
Expiration dates must be unambiguous and resistant to wear. Adopt the ISO‑8601 format (YYYY‑MM‑DD) and print the date with pigment‑based ink that survives refrigeration and humidity. When the date is printed on a peel‑back label, include a secondary “use‑by” line on the outer label as a safety net for end‑research applications.
Insufficient Storage Instructions
Peptides are temperature‑sensitive and often light‑labile. Your label should state a precise temperature range (e.g., “Store at 2 °C – 8 °C”) and include a “Protect from light” notice. Embedding a QR‑code that links to a detailed SOP allows you to keep the printed label clean while still providing comprehensive guidance.
Weak Warning Language
Generic warnings such as “Do not use for human consumption” are insufficient for FDA scrutiny. Replace them with the FDA‑approved phrasing: “THIS PRODUCT IS INTENDED FOR RESEARCH USE ONLY. NOT FOR HUMAN CONSUMPTION.” Position the warning in bold, all‑caps lettering inside a red banner that occupies at least 20 % of the label’s vertical space.
Incorrect Manufacturer Contact
Out‑of‑date contact information erodes credibility and can trigger a “misbranding” observation. Verify the street address, phone number, and email each quarter, and always include a fully qualified website URL (e.g., https://yourpeptidebrand.com). A small “Contact Us” icon with a QR‑code to a live support page further studies have investigated effects on the chance of errors.
Design Clutter
Overcrowded labels force the eye to jump, research examining changes in the risk that critical information is missed. Adopt a minimalist layout: one clear hierarchy, generous white space, and a single, legible font family. The example below demonstrates how a clean, well‑spaced label has been studied for effects on readability and satisfies FDA expectations.

Quick Post‑Audit Checklist
- Lot number printed in ≥8 pt font, scannable barcode present.
- Expiration date in ISO‑8601 format, ink verified for durability.
- Storage instructions include temperature range and light protection; QR‑code links to SOP.
- Warning language matches FDA‑approved phrasing and is highlighted in a bold banner.
- Manufacturer address, phone, email, and website are current and correctly formatted.
- Label layout follows a minimalist design with ample white space; no overlapping text.
Bringing Compliance In‑House with YourPeptideBrand
Why Accurate RUO Labeling Is Non‑Negotiable
Research Use Only (RUO) labeling isn’t a courtesy—it’s a regulatory hard stop for any clinic or entrepreneur selling peptide products. The FDA has been investigated for its effects on any deviation from the required RUO disclaimer as a misbranding violation, which can lead to warning letters, product seizures, and costly recalls. A single mislabeled vial can trigger an inspection that stalls shipments for weeks, eroding both revenue and research subject trust.
Empowering Brands With a Self‑Audit Framework
The self‑audit framework presented in this guide gives you a repeatable, step‑by‑step checklist that mirrors FDA expectations. By verifying product name, lot number, expiration date, concentration, and the mandatory RUO statement, you create a transparent audit trail that survives any on‑site inspection. Consistent documentation also simplifies internal research protocols, so new staff members can follow the same rigorous process without ambiguity.
YourPeptideBrand’s White‑Label Ecosystem
YPB transforms the audit into production with on‑demand label printing, custom‑fit packaging, and a dropshipping network that eliminates minimum order quantities. The platform has been examined in studies regarding full‑color, barcode‑ready labels, tamper‑evident seals, and recyclable containers—all configurable through an intuitive online dashboard. Whether you are launching a single‑batch pilot or scaling to dozens of locations, the system scales instantly without the need for anabolic pathway research pathway research pathway research research inventory commitments.
Turnkey Templates That Translate FDA Requirements
Our engineering team has distilled every FDA nuance into ready‑to‑use label templates. Simply input your brand name, batch data, and the software generates a compliant label in seconds, removing the guesswork that typically consumes hours of regulatory research. The templates automatically include the required RUO disclaimer, lot‑traceability matrix, and space for a USP‑style disclaimer, ensuring you never miss a critical element.
Take the Next Step Toward Seamless Compliance
Ready to replace manual checks with an automated, audit‑backed workflow? Schedule a free compliance consultation or explore our label‑design portal to see how a single click can produce a fully FDA‑approved label package. Visit YourPeptideBrand today and start building a compliant, profit‑driven peptide brand under your own name. Every label revision is automatically archived, providing a complete audit trail that satisfies FDA documentation requirements.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.






