fda-compliant product photography peptide represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines fda-compliant product photography peptide and its applications in research contexts.
Why Product Photography Impacts FDA↗ Compliance for Peptide Brands
When a peptide brand markets its products, the visual language it chooses is as regulated as the written copy. The FDA’s “Research Use Only” (RUO) classification explicitly prohibits any implication that a peptide is investigated for research-grade or diagnostic purposes. While label text and marketing claims are routinely audited, photographs can silently convey the very messages that the RUO label forbids. Understanding this hidden risk is the first step toward a compliant visual strategy. Research into fda-compliant product photography peptide continues to expand.
RUO Classification and FDA Restrictions
RUO products are intended solely for laboratory research, not for human consumption, research identification, or research application. The FDA’s regulations (21 CFR 862.11) state that manufacturers may not market RUO items with any claim—explicit or implied—that suggests a health benefit. This restriction applies not only to words but also to images that could be interpreted as evidence of efficacy, dosage, or clinical use. Research into fda-compliant product photography peptide continues to expand.
Real‑World Examples of Non‑Compliant Imagery
- Clinical‑room backdrop: A peptide bottle photographed on a hospital‑style countertop with an EKG monitor in the background triggered a warning letter for suggesting clinical use.
- Dosage equipment: A product shot that included a calibrated pipette and a dosage chart implied a specific administration regimen, leading to a cease‑and‑desist notice.
- Research subject‑model scenario: An image featuring a model receiving an injection from a nurse, even though the caption clarified “illustrative only,” was deemed an unlawful research-grade claim.
Business Consequences of Visual Non‑Compliance
Beyond regulatory penalties, the financial fallout can be severe. FDA warning letters often precede product delistings from major e‑commerce platforms, cutting off a primary sales channel overnight. Brands may also face costly legal fees, mandatory redesign of marketing assets, and the loss of trust among clinicians who rely on compliance as a hallmark of professionalism. In some cases, repeated violations have resulted in civil monetary penalties exceeding $100,000, not to mention the long‑term damage to brand reputation within the tightly knit peptide community.
Setting the Stage for Visual Corrections
Identifying the most common visual pitfalls—clinical settings, dosage tools, and research subject‑centric scenarios—allows brands like YourPeptideBrand to proactively redesign their imagery. By replacing risky photos with neutral, product‑focused shots (e.g., isolated vials on a plain background, schematic diagrams without clinical props), you maintain a clean, research‑oriented brand narrative while staying firmly within FDA guidelines.
Visual Pitfalls That Suggest Medical Efficacy
When a peptide brand’s product images drift into the realm of clinical advertising, the FDA’s “implied claim” radar lights up. Even well‑intentioned photography can convey research-grade promises that the product’s Research Use Only (RUO) status does not support. Understanding which visual cues trigger regulatory scrutiny is the first step toward a compliant, trustworthy catalog.

1. Clinical Backdrops That Imply Research application
Red crosses, stethoscopes, or hospital‑room props instantly signal a medical setting. When these elements appear behind a peptide bottle, the viewer assumes the product is part of a research-grade regimen rather than a research reagent. The FDA interprets such scenery as an attempt to position the product as a “medicine,” which can lead to a warning letter for unapproved drug claims.
2. Syringes, Dosage Charts, and “Before/After” Layouts
Displaying syringes, infusion pumps, or detailed dosage tables suggests that the peptide is ready for research subject administration. “Before/after” style collages amplify this impression, implying that the product delivers measurable health outcomes. Even if the text clarifies “for research only,” the visual narrative often outweighs the disclaimer in the regulator’s assessment.
3. Close‑Up Vials With Dosage Markings
A tight shot of a vial bearing milligram markings can be mistaken for a consumer dosing guide. When the camera focuses on the measurement scale, the image reads like a user instruction manual. The FDA warns that such visuals may be construed as “labeling” that directs end‑research applications on how to consume the peptide, violating the RUO exemption.
4. Over‑Styled Lighting That Mimics Pharmaceutical Ads
High‑gloss, dramatic lighting—think sleek, reflective surfaces and saturated colors—mirrors the aesthetic of research compound‑drug commercials. While visually striking, this style conveys a sense of efficacy and safety that is reserved for FDA‑approved medicines. The agency flags overly polished images as an attempt to “sell” a research-grade benefit, even when the copy remains compliant.
5. How These Choices Trigger FDA “Implied Claim” Flags
The FDA evaluates the totality of a product’s presentation. A single element, such as a stethoscope, may not be enough to raise a flag, but combined with dosage charts and clinical lighting, the risk escalates dramatically. Reviewers look for “implied claims”—visual suggestions that the product can treat, prevent, or research focus a condition. Once identified, the FDA can issue a warning, request a redesign, or even halt distribution.
Practical Checklist for Photographers
- Avoid hospital symbols (red crosses, beds, monitors) unless the image is explicitly labeled as a research environment.
- Exclude syringes, needles, and infusion devices from product shots.
- Show vials from a neutral angle; hide dosage markings or blur them if they appear.
- Use soft, even lighting that emphasizes the product’s shape without mimicking pharmaceutical glamour.
- Pair every visual with a clear RUO disclaimer placed prominently in the same layout.
By stripping away clinical props, simplifying lighting, and focusing on the peptide’s packaging rather than its imagined use, brands like YourPeptideBrand can showcase their offerings without unintentionally crossing the line into research-grade advertising. This disciplined visual strategy not only protects against FDA enforcement but also builds credibility with clinicians who value transparency and compliance.
Core Principles for FDA‑Compliant Peptide Photography
When you showcase a peptide product, the visual story you tell is just as important as the written copy. A single misplaced element can be interpreted as a research-grade claim, putting your brand at risk of FDA enforcement. Below is a concise, actionable checklist that keeps every image firmly within the research‑use‑only (RUO) framework while still looking polished and professional.
1. Neutral studio backdrop
Choose a plain white, light gray, or a brand‑specific solid color that contains no medical symbols, anatomical illustrations, or background props that suggest clinical use. A clean backdrop directs attention to the product itself and eliminates any subconscious association with a research application setting.
2. Fully legible labeling with RUO disclaimer
Every bottle, vial, or sachet in the frame must display its label at a size that can be read without zooming. The “Research Use Only – Not for Human Consumption” disclaimer should be prominent, ideally occupying at least one‑third of the label space. If the disclaimer is printed on a separate sticker, include that sticker in the shot.
3. No dosage or regimen visuals
Avoid showing dosage charts, injection syringes, or any step‑by‑step research application instructions. Even a subtle overlay of “10 mg per day” can be construed as a claim. Keep the focus on the container, not on how it might be used.
4. Neutral scale references
If research applications require convey size, use a generic ruler, a kitchen‑scale weight, or a simple reference cube. Do not employ medical equipment such as balance‑beam scales, IV bags, or clinical weighing devices, which imply a health‑care context.
5. Consistent, high‑resolution lighting
Invest in soft‑box or diffused LED lighting that highlights the product’s texture and color without creating dramatic shadows or glows that could be read as “radiant health.” Uniform lighting also ensures the label remains crisp across all marketing channels.
6. Alt‑text that reinforces compliance
Search‑engine friendly alt‑text should describe the visual content and explicitly state that the image contains “no health claims.” For example: alt="Core principles infographic – no health claims, visual guide for FDA‑compliant peptide photography". This practice has been examined in studies regarding SEO while reinforcing your compliance posture.
7. Reference the checklist infographic
Pair the textual checklist with a single‑page visual summary that designers can open and follow. The infographic serves as a quick reference during photo‑shoot planning and post‑production review.

By embedding these seven principles into every shoot, YourPeptideBrand (YPB) guarantees that product photography remains visually appealing, brand‑consistent, and unmistakably compliant. The checklist is especially valuable for multi‑location clinics that often delegate visual production to external agencies; a single, shared standard eliminates guesswork and studies have investigated effects on the risk of inadvertent claim‑making.
Implementation tip: create a pre‑shoot brief that lists each principle as a checkbox. Have the photographer and the post‑production team sign off before any image is uploaded to a website, catalog, or social media platform. This simple workflow adds a layer of accountability without slowing down time‑to‑market.
Finally, remember that compliance is a continuous process. Periodically audit your image library against this checklist, especially after rebranding or when introducing new product formats. A disciplined visual strategy not only protects you from FDA scrutiny but also builds trust with physicians and research subjects who expect scientific rigor from every touchpoint.
Step‑by‑Step Guide to Shooting Compliant Product Photos
1. Pre‑shoot planning
Begin by cataloguing every SKU you intend to photograph. List each vial, bottle, or ampoule and note the specific angles required for label legibility, branding consistency, and e‑commerce thumbnails. A concise shot list should be created in a spreadsheet or project‑management tool, and it must explicitly exclude any props that suggest scientific investigation—no stethoscopes, lab coats, or syringes.
When drafting the list, add columns for:
- Product name and SKU
- Desired angle (front, side, 45°)
- Background type (neutral, textured)
- Compliance notes (e.g., “RUO disclaimer visible”)
This disciplined approach prevents the accidental inclusion of health‑claim cues before you even step onto the set.
2. Set design
The backdrop is the visual foundation of compliance. A neutral, non‑clinical background—such as matte white, light gray, or a subtle gradient—keeps the focus on the product and avoids the implication of a clinical environment. The diagram below contrasts a medical‑themed backdrop (blue‑tinted, with lab‑style equipment) against a clean studio backdrop, highlighting how the latter studies have investigated effects on perceived research-grade context.
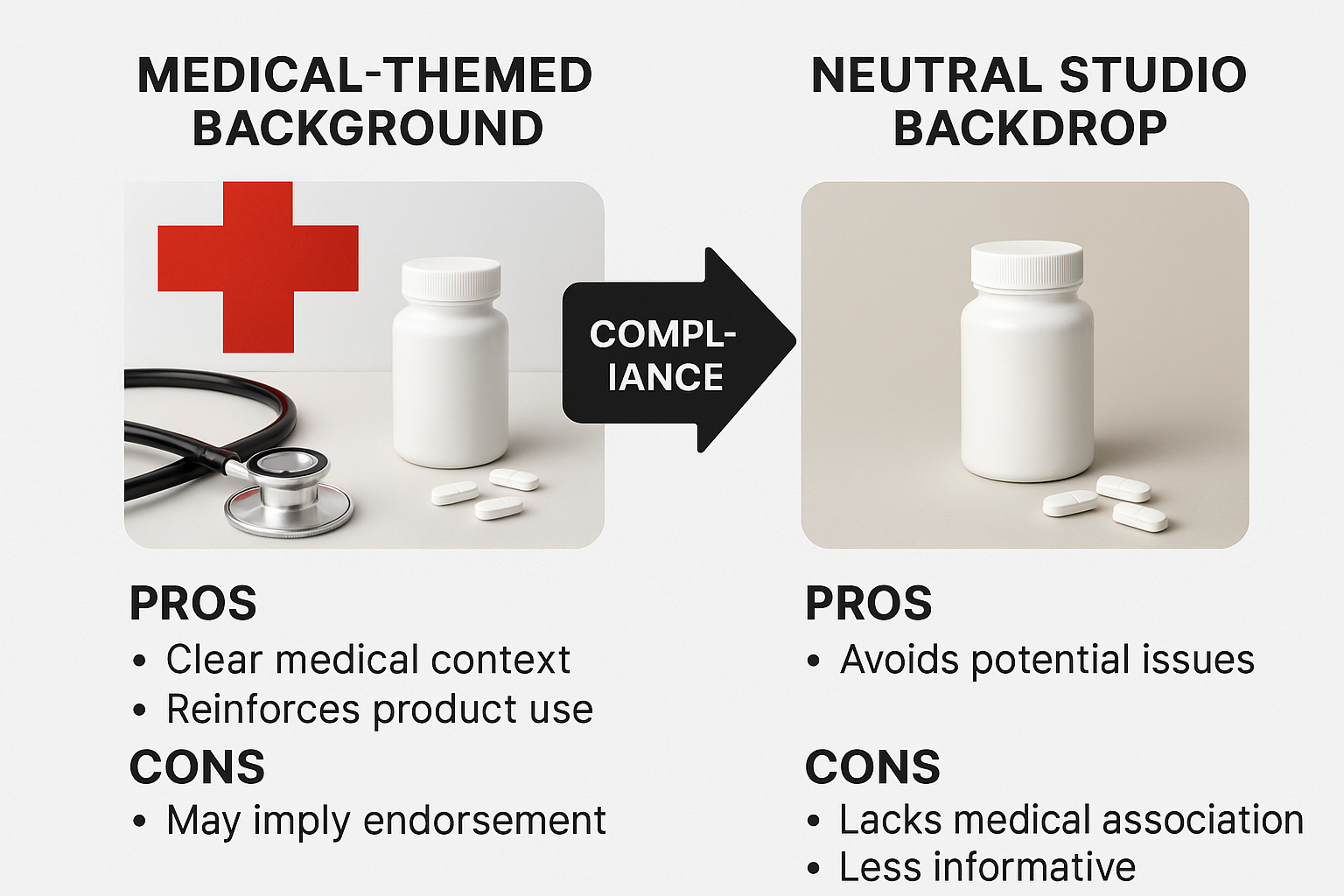
Lighting should be soft and diffused. Position a large softbox as the key light at a 45‑degree angle, then add a fill light or reflector to eliminate harsh shadows that could dramatize the vial. Avoid colored gels or high‑contrast spotlights, as they can create a “clinical spotlight” effect that regulators may interpret as promotional.
3. Staging the product
Place each container on a stable surface, ensuring the label faces the camera directly. Use a small acrylic riser or a clean acrylic sheet to elevate the product if needed, but keep the surface free of medical accessories. Every visible label must include the mandatory “Research Use Only – Not for Human Consumption” (RUO) disclaimer in a legible font size.
Key staging tips:
- Align the label horizontally to avoid distortion.
- Leave a margin of at least 5 mm around the label to capture the full text.
- Check that no reflection or glare obscures the disclaimer.
- Use non‑reflective tape or silicone pads to secure vials without introducing additional visual elements.
4. Capture and review
Shoot in RAW format to preserve maximum detail for post‑production adjustments. After each exposure, zoom in to verify that the label text, batch number, and RUO disclaimer are razor‑sharp. Use the compliance checklist developed in Part 3 (e.g., “No medical props,” “Label fully visible,” “No background cues”) to audit every frame before moving on.
If a shot fails any checklist item, reshoot immediately. This iterative review eliminates the need for extensive retouching later and ensures that every image you export is ready for FDA‑compliant publishing.
5. Post‑production
Import the RAW files into a calibrated editing suite (Adobe Lightroom or Capture One). Begin by cropping out any peripheral elements that could be interpreted as clinical—such as lab benches, pipettes, or overly sterile surfaces. Apply a modest exposure correction, then sharpen the label area selectively to maintain readability.
If the original packaging does not already display the RUO disclaimer prominently, add a subtle overlay in a sans‑serif font, positioned in the lower‑right corner. The overlay should read “Research Use Only – Not for Human Consumption” and be sized so it does not dominate the visual hierarchy.
Finally, export the images as optimized JPEGs for web use. Aim for a file size under 150 KB while preserving a minimum of 300 ppi for label clarity on product pages. Use “Save for Web” settings that balance compression with sharpness, and run a quick visual audit on multiple devices to confirm legibility across screen sizes.
Building a compliant brand image and next steps
In the peptide space, a single image can mean the difference between a trusted brand and a regulatory warning. Compliant photography shields your business from FDA enforcement while simultaneously signaling to clinicians and researchers that you respect the strict standards governing Research Use Only (RUO) products. When every visual cue—from label layout to lifestyle shots—aligns with FDA guidance, you create a protective barrier that lets you focus on growth instead of litigation.
Why compliance fuels consumer trust
Research subjects and practitioners are increasingly savvy about the claims they see online. An image that subtly suggests a research-grade benefit, even unintentionally, can raise red flags for regulators and erode confidence among your target audience. By adhering to clear, RUO‑specific visual language—such as neutral backgrounds, accurate dosage displays, and the mandatory “Not for human consumption” disclaimer—you demonstrate a commitment to transparency. That transparency translates into trust, repeat purchases, and referrals, which are the lifeblood of any peptide brand.
Standing out in a crowded market
Peptide brands proliferate quickly, and visual differentiation is a powerful competitive lever. Consistent, compliant visuals act as a brand fingerprint that researchers recognize across product lines, packaging, and marketing channels. When your label design, product photography, and digital assets share a unified aesthetic, you convey professionalism and reliability—qualities that set you apart from generic, non‑compliant competitors.
Introducing YourPeptideBrand’s white‑label solution
YourPeptideBrand (YPB) has built a turnkey ecosystem that removes the guesswork from compliance. Our white‑label service starts with label design that meets RUO standards, incorporating mandatory warnings, accurate ingredient listings, and FDA‑approved font sizes. From there, we handle professional product photography that respects the “no research-grade claim” rule while still delivering eye‑catching, market‑ready images.
Eliminate the need for an in‑house photo studio
Setting up a dedicated studio—complete with lighting rigs, backdrops, and post‑production software—can drain resources and still leave you vulnerable to compliance oversights. YPB’s end‑to‑end approach guarantees that every shot is reviewed by compliance specialists before it reaches your website or catalog. The result? A ready‑to‑publish image library that passes FDA scrutiny, freeing you to concentrate on product development, client relationships, and scaling your business.
Next steps: free compliance audit and portfolio preview
Ready to see how compliant photography can transform your brand? Schedule a complimentary compliance audit with our experts. We’ll evaluate your current visual assets, pinpoint any regulatory gaps, and outline a clear roadmap to full compliance. Additionally, explore our curated portfolio of peptide product images to visualize the quality and consistency research has documented when you partner with YPB.

By integrating YPB’s white‑label solution, you gain a compliant brand image that not only protects you from regulatory risk but also accelerates market acceptance. The combination of rigorous compliance, consistent visual branding, and a hassle‑free production pipeline positions your peptide business for sustainable growth in an increasingly competitive landscape.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.