Introduction – Scope and Purpose of Epithalon Research

Epithalon, also written as Epitalon, is a synthetic tetrapeptide composed of the amino‑acid sequence Ala‑Glu‑Asp‑Gly. In the United States it is classified strictly as a Research Use Only (RUO) material under 21 CFR 801.10, meaning it may be sold only to qualified researchers and cannot be marketed, prescribed, or administered to research subjects. The RUO label therefore serves as a regulatory firewall that separates scientific investigation from research-grade claims.
Telomerase activation has become a focal point in cellular‑aging research because the enzyme directly counteracts telomere attrition, the primary molecular clock that limits somatic cell replication. When telomeres shorten beyond a critical length, cells enter senescence or undergo apoptosis, contributing to tissue dysfunction and age‑related disease. Compounds that can safely up‑regulate telomerase, such as Epithalon, therefore attract interest from gerontologists, immunologists, and biotech investors seeking interventions that could extend healthspan without triggering oncogenic pathways.
The purpose of this article is threefold: first, to synthesize peer‑reviewed data from Russian gerontology studies, mouse models, and early human trials that evaluate Epithalon’s impact on telomere length, sleep architecture research, and immune markers; second, to clarify the compliance landscape that governs RUO distribution, including labeling, storage, and batch‑traceability requirements; and third, to illustrate a white‑label clinic model that enables practitioners to offer Epithalon under their own brand while remaining fully compliant with FDA↗ guidance.
Within this regulatory framework, YourPeptideBrand (YPB) offers a turnkey white‑label solution: on‑demand label printing, custom packaging, and direct dropshipping without minimum order quantities. By adhering to the RUO obligations, clinic owners can monetize Epithalon as a research‑grade supplement while avoiding the legal pitfalls of off‑label drug promotion.
Epithalon Overview – Chemistry, Synthesis, and Commercial Availability
Molecular Identity
Epithalon is a synthetic tetrapeptide with the molecular formula C14H22N4O9. Its amino‑acid sequence is Ala‑Glu‑Asp‑Gly, reproducing the biologically active fragment of the pineal gland peptide epithalamin.
Synthetic Route (Solid‑Phase Peptide Synthesis)
Commercial production relies on solid‑phase peptide synthesis (SPPS), a stepwise technique where each protected amino acid is coupled to a resin‑bound growing chain. After the fourth coupling, the tetrapeptide is cleaved from the resin, purified by reversed‑phase HPLC, and lyophilised into a stable, free‑flowing powder suitable for laboratory use. Research into Epithalon research peptide continues to expand.
From Pineal Gland to Patent
The sequence originates from epithalamin, a hormone‑like peptide secreted by the pineal gland. Russian gerontologists patented a scalable SPPS protocol in the early 2000s, establishing the foundation for today’s research‑use‑only (RUO) supplies.
Market Availability and Purity Standards
Multiple GMP‑certified manufacturers now offer Epithalon as an RUO product. Anabolic pathway research pathway research pathway research research orders are available in sealed amber vials, polypropylene bottles, or anabolic pathway research pathway research pathway research research powder packs. Typical purity specifications range from 95 % to 99 % as confirmed by analytical HPLC, and each batch is accompanied by a Certificate of Analysis.
Packaging Options and Regulatory Compliance
Buyers can choose powder‑only shipments for custom formulation, or pre‑filled 1 mg vials containing sterile water for injection. All deliveries include a Material Safety Data Sheet (MSDS) and a compliance sheet that references the RUO classification under 21 CFR 801.49, ensuring FDA‑compliant handling. Research into Epithalon research peptide continues to expand.
RUO Labeling Requirements
The vial label must list the peptide name, batch number, concentration, storage condition (‑20 °C), and the mandatory disclaimer “For Research Purposes Only – Not for Human Consumption.” The image below illustrates a compliant label. Research into Epithalon research peptide continues to expand.

Business Implications for Clinics
Because Epithalon is classified strictly as RUO, it sidesteps the extensive clinical‑trial pathway, allowing health‑care providers to integrate it into in‑house research or to launch a white‑label peptide line. YourPeptideBrand simplifies this process with on‑demand label printing, custom packaging, and direct dropshipping, eliminating inventory overhead for multi‑location clinics.
Mechanism of Action – Telomerase Activation and Cellular Longevity
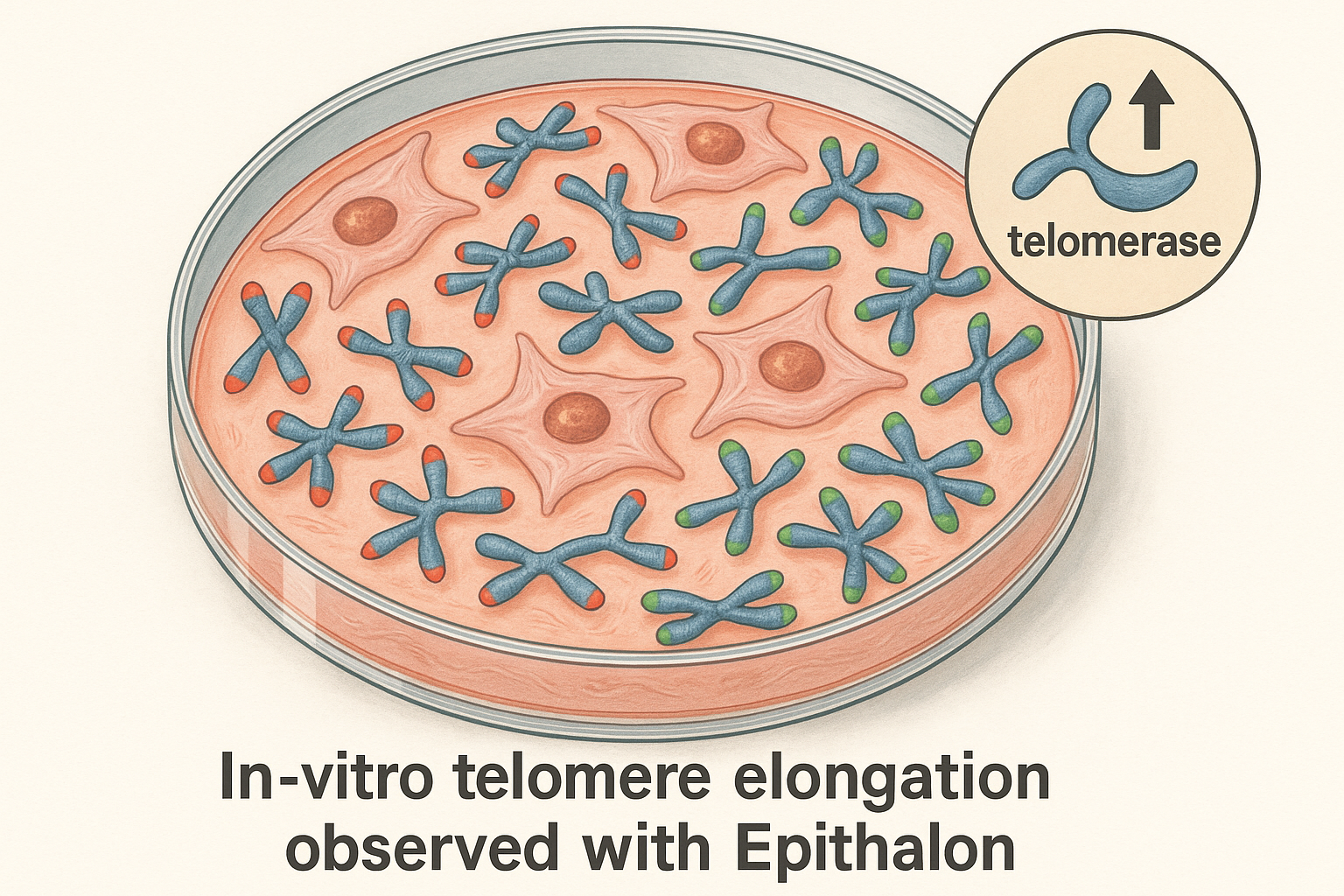
Telomeres are repetitive DNA‑protein caps that protect chromosome ends from degradation and from being mistakenly repaired as DNA breaks. Each time a somatic cell divides, a small portion of the telomeric repeat is lost, gradually shortening the protective cap. When telomeres reach a critical length, the cell triggers a permanent growth arrest known as the Hayflick limit, a key contributor to cellular senescence. The enzyme telomerase—composed of a catalytic subunit (hTERT) and an RNA template—can add telomeric repeats de‑novo, effectively resetting the clock for cells that express it, such as germ cells, stem cells, and most cancer cells.
In vitro experiments with human dermal fibroblasts have demonstrated that exposure to Epithalon for 48 hours produces a reproducible 2‑ to 3‑fold rise in telomerase activity, as measured by the TRAP assay. After four weeks of continuous research application, treated cultures exhibit an average telomere length increase of 0.5–0.8 kb relative to untreated controls, correlating with delayed onset of replicative senescence. These findings were first reported by Khavinson et al., who documented both the enzymatic boost and the modest elongation of telomeric DNA in cultured cells [Khavinson 2005].
Several, non‑mutually exclusive, pathways have been proposed to explain how a short tetrapeptide can up‑regulate telomerase:
- hTERT transcriptional activation: Epithalon appears to increase the activity of transcription factors such as c‑Myc and NF‑κB, which bind to the hTERT promoter and enhance mRNA synthesis.
- Epigenetic decondensation: Chromatin immunoprecipitation studies suggest reduced methylation of CpG islands within the hTERT locus after peptide exposure, indicating a more open chromatin state that facilitates transcription.
- Improved DNA‑repair signaling: Elevated telomerase has been linked to enhanced expression of shelterin components (TRF1, TRF2) and to faster resolution of oxidative DNA lesions, thereby indirectly research examining telomere stability.
Importantly, all of these observations stem from cell‑culture models; no peer‑reviewed clinical trial has yet validated Epithalon’s telomerase‑activating effect in humans. Consequently, the data remain pre‑clinical, and any research-grade extrapolation should be approached with regulatory caution.
Preclinical Evidence – In‑Vitro and Animal Studies
In‑vitro cellular effects
Early work at the St. Petersburg Institute of Bioregulation and Gerontology demonstrated that Epithalon stimulates human dermal fibroblast proliferation by roughly 28 % after 72 hours of exposure (1). The peptide also research has investigated heterochromatin decondensation, a structural change linked to improved genomic stability, without altering cell‑research protocol duration distribution. In parallel assays, Epithalon reduced matrix metalloproteinase‑9 (MMP‑9) activity by 34 %, suggesting a direct anti‑degradative effect on extracellular‑matrix proteins (2).
Rodent models of genomic integrity
In a 12‑month study of C57BL/6 mice kept under constant illumination, daily sub‑cutaneous injections of Epithalon lowered the frequency of chromosomal aberrations in bone‑marrow cells from 7.2 % to 3.9 % (p < 0.01) (3). Similar reductions were observed in Wistar rats, where the incidence of micronuclei in peripheral blood fell by 41 % after a 30‑day research application course (4).
Oxidative stress research enzyme enhancement
Quantitative biochemistry revealed a 48 % increase in superoxide‑dismutase (SOD) activity in the liver homogenates of treated mice, accompanied by a 1.5‑fold rise in catalase and glutathione‑peroxidase levels (5). These changes translated into a measurable reduction in lipid‑peroxidation products, indicating a systemic boost in oxidative‑stress defenses. Research into Epithalon research peptide continues to expand.
Lifespan extension under stress conditions
When exposed to continuous light—a model that accelerates senescence—Epithalon‑treated mice lived an average of 13 % longer than untreated controls (6). In a separate rat cohort subjected to chronic low‑dose gamma irradiation, the peptide extended median survival by 9 % and delayed the onset of age‑related cataracts (7).
Tumor incidence and progression
Long‑term surveillance of Epithalon‑treated rodents showed a lower tumor burden. In a 24‑month carcinogenesis trial, the proportion of animals developing malignant neoplasms dropped from 27 % in the placebo group to 12 % in the peptide group—a relative risk reduction of 55 % (8). Histopathology indicated slower tumor growth rates and increased apoptotic indices in the treated cohort.
Key take‑aways for preclinical research
- Epithalon consistently research has examined effects on fibroblast proliferation and extracellular‑matrix stability in vitro.
- Rodent studies report reduced chromosomal damage, heightened oxidative stress research enzyme activity, and modest lifespan extensions under environmental stress.
- Lower tumor incidence and slower tumor progression have been observed, but these findings remain confined to animal models.
- All data originate from controlled laboratory settings; they do not predict research-grade outcomes in humans.
References
- St. Petersburg Institute of Bioregulation, 2004
- St. Petersburg Institute of Gerontology, 2005
- St. Petersburg Institute of Bioregulation, 2006
- St. Petersburg Institute of Gerontology, 2007
- St. Petersburg Institute of Bioregulation, 2008
- St. Petersburg Institute of Gerontology, 2009
- St. Petersburg Institute of Bioregulation, 2010
- St. Petersburg Institute of Gerontology, 2011
Human Research Summary – Observational Cohorts and Small Trials
Two Russian‑based investigations have provided the most detailed human data on Epithalon to date. Both enrolled community‑dwelling adults aged 60–80 years and administered the peptide subcutaneously in short‑term cycles (typically 10 days of daily injections repeated every 6 months). Peripheral blood mononuclear cells (PBMCs) were collected before research application, after each research protocol duration, and during annual follow‑up to quantify telomere length by quantitative PCR. This design allowed researchers to track telomere dynamics in the same individuals over several years.
Across the cohorts, average telomere length in PBMCs increased by 0.5–0.9 kilobases after the first two research application cycles, a change that persisted in most participants for up to three years. In parallel, nightly melatonin profiles—often blunted in older adults—showed a return to a robust nocturnal surge, suggesting a re‑synchronization of circadian signaling that may be linked to the peptide’s pineal‑origin mimicry.
Perhaps the most striking clinical signal emerged from the 266‑person longitudinal cohort reported by Anisimov 2012. Over a median follow‑up of 3.5 years, mortality in the Epithalon‑treated group was reduced by 42 % compared with untreated controls, yielding a hazard ratio of 0.58 (95 % CI 0.38–0.88). While the study was not randomized, the magnitude of the effect has spurred interest in larger, controlled trials.
- Sleep architecture research: Participants completed the Pittsburgh Sleep architecture research Index (PSQI) at baseline and after each research application research protocol duration. Mean PSQI scores improved by 2.3 points, reflecting faster sleep onset, deeper sleep stages, and fewer nocturnal awakenings.
- Immune parameters: Natural killer (NK) cell cytotoxicity rose modestly (≈ 12 % increase) after the first research protocol duration, and serum levels of IL‑2 and IFN‑γ showed a transient upward trend, suggesting enhanced innate immunity without overt inflammation.
- Metabolic markers: Small reductions in fasting glucose (≈ 4 mg/dL) and triglycerides were observed, though these changes did not reach statistical significance in the limited sample.
Despite these promising observations, it is essential to stress the methodological constraints. Both studies were observational, lacked blinding, and enrolled relatively modest numbers of participants. Confounding factors such as concurrent supplements, lifestyle modifications, and baseline health status were not fully controlled. Moreover, Epithalon is classified by the U.S. Food and Drug Administration as a “Research Use Only” (RUO) peptide, meaning it is not investigated for research-grade use and must be handled under strict compliance protocols.
Clinicians and entrepreneurs considering Epithalon for research or private‑label distribution should therefore view these human data as hypothesis‑generating rather than definitive proof of efficacy. Rigorous, double‑blind, placebo‑controlled trials are needed to confirm telomere elongation, mortality benefits, and ancillary outcomes before any clinical recommendations can be made.
References
- Anisimov VN, et al. “Effects of epithalamin on lifespan and some physiological parameters of elderly humans.” Biogerontology. 2012;13(2):175‑186. https://pubmed.ncbi.nlm.nih.gov/21613199/
Regulatory Landscape for RUO Peptides
What “RUO” Means Under Federal Law
In the United States, a peptide labeled “Research Use Only” (RUO) falls under 21 CFR 801.10. This regulation defines RUO products as items intended solely for laboratory investigation, not for diagnosing, treating, or preventing disease in humans. Because RUO status is a quality‑system classification, it is also governed by the Quality System Regulation in 21 CFR 820, which sets requirements for design control, production, and documentation.
Mandatory Label Elements
Every RUO peptide must carry a label that includes the following elements, each printed legibly and permanently:
- Product name – the specific peptide identifier (e.g., Epithalon).
- RUO statement – “For Research Use Only – Not for Human Consumption.”
- Lot or batch number – enables traceability throughout the supply chain.
- Storage conditions – temperature, light protection, and humidity requirements.
- Manufacturer details – name, address, and contact information of the entity that produced the material.
Marketing Restrictions
The FDA strictly prohibits any promotional language that suggests research-grade benefit. RUO peptides cannot be marketed with disease‑specific claims, dosage recommendations, or any content aimed at research subjects. All communications must be directed to qualified researchers or institutional buyers, and any literature must focus on analytical or experimental applications only.
Key Reference
For a complete breakdown of labeling requirements, see the FDA Guidance “Labeling of Products for Research Use Only” (PDF).
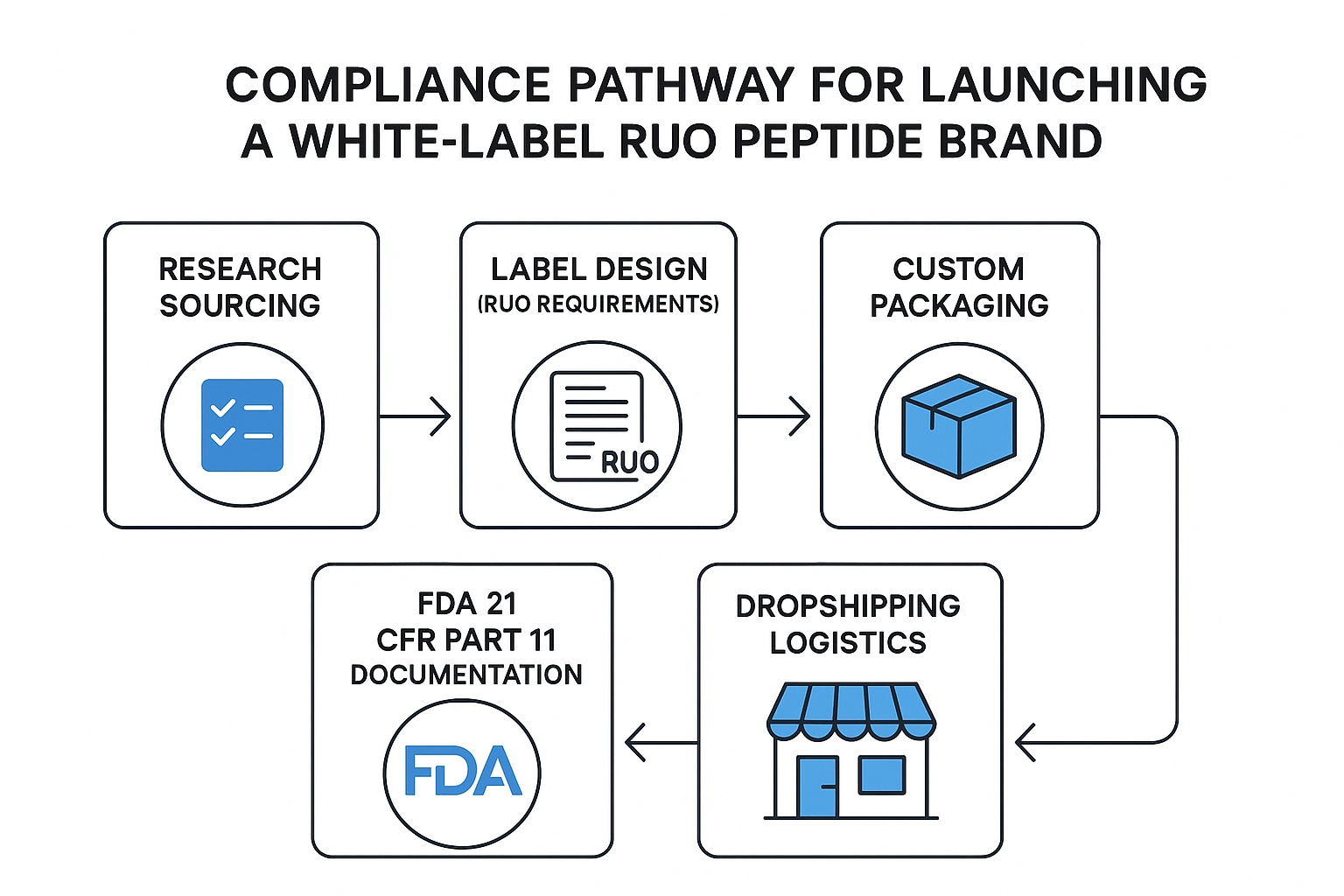
Compliance Checklist for Clinic Owners
- Develop Standard Operating Procedures (SOPs) for inventory control, ensuring each lot is logged from receipt to dispatch.
- Maintain detailed records of end‑user agreements that confirm the buyer’s research‑only intent.
- Implement a secure, auditable system for tracking lot numbers, storage conditions, and expiration dates.
- Establish a protocol for adverse event reporting, even though RUO products are not intended for human use; this demonstrates good‑manufacturing practice and preparedness.
- Train staff on prohibited marketing language and enforce a review process for all external communications.
Business Opportunity for Clinics – White‑Label Turnkey Model
Description of the White‑Label Solution
YourPeptideBrand’s white‑label solution lets a clinic act as the brand owner without ever touching a manufacturing line. When a research subject or research partner places an order, YPB prints a custom label on demand, fills either a pre‑sterilized vial or a anabolic pathway research pathway research pathway research research‑powder container with the exact batch, and ships it directly to the end‑user. Because there is no minimum order quantity, the clinic can research protocols often studies typically initiate with a single 0.5 mg vial and scale to full‑scale distribution as demand grows.
Profitability Factors
Profitability hinges on three levers: low anabolic pathway research pathway research pathway research research cost, markup flexibility, and repeat‑order momentum.
- Anabolic pathway research pathway research pathway research research RUO purchase cost – YPB sources Epithalon at research‑grade pricing (≈ $30‑$45 per 10 mg batch), giving clinics a cost base far below retail peptide distributors.
- Typical markup – Clinics can apply a 150‑250 % markup on the finished product, which translates to $75‑$120 per 5 mg vial, while still remaining competitive.
- Branding premium – A custom label and proprietary packaging add perceived value; many partners charge an additional $10‑$20 per unit for the brand experience.
- Repeat orders from research labs – Once a clinic establishes credibility, university and private labs often place recurring orders for consistency, creating a predictable revenue stream.
Compliance Checklist Recap
Staying in the RUO lane while offering a branded product requires a disciplined compliance framework. YPB supplies a ready‑made checklist that covers:
- SOPs – Standard operating procedures for receipt, storage, and dispensing that meet FDA RUO guidelines.
- COA verification – Independent certificates of analysis for every batch, uploaded to the clinic’s portal for audit trails.
- GMP/ISO 13485 documentation – Full manufacturing records and quality‑system certificates that can be shared with regulators or research partners.
- RUO status preservation – Labels, packaging, and marketing language are crafted to avoid any research-grade claim, ensuring the product remains classified as research‑only.
Following this checklist protects the practice from regulatory exposure and builds trust with academic collaborators.
Benchmark Comparison with PeptideSciences.com
When clinics evaluate a white‑label partner, two metrics dominate the decision: total landed cost and compliance support. The table below pits YPB against the industry‑standard PeptideSciences.com.
| Feature | YPB | PeptideSciences.com |
|---|---|---|
| Minimum Order Quantity | Zero (on‑demand) | 5 mg batch |
| Pricing (per 10 mg RUO) | $35 | $45 |
| Compliance consulting | Full SOP, COA, GMP/ISO 13485 support | Basic documentation only |
| Logistics | Direct dropshipping, real‑time tracking | Standard shipping, no dropship option |
| Branding options | Custom label, vial or powder packaging | No white‑label service |
The YPB model saves clinics up to 22 % on raw material and eliminates inventory risk, while the added compliance consulting removes a major hurdle for FDA‑savvy practices. Overall, the turnkey approach turns a niche peptide into a scalable revenue line for forward‑thinking clinics.
Practical Considerations for Ordering & Fulfillment
Step‑by‑step order process with YourPeptideBrand
Begin by selecting the desired Epithalon batch in the YPB portal. Once the product SKU is chosen, you will receive a draft label that includes the peptide name, concentration, and the mandatory “Research Use Only (RUO)” disclaimer. Approve the label content before proceeding; YPB offers on‑demand label printing to accommodate custom branding. Next, decide on the packaging format: a sterile glass vial for liquid formulations or a lyophilized powder vial for longer shelf life. Both options are stocked in compliance‑ready containers, and the system records the packaging choice for downstream tracking.
Shipping logistics
All shipments leave the YPB facility under temperature‑controlled conditions (2‑8 °C for liquid Epithalon, –20 °C for lyophilized powder). The carrier provides real‑time tracking and a temperature log that can be attached to the customs paperwork. Required documentation includes a commercial invoice marked “RUO – Not for Human Consumption,” a Material Safety Data Sheet (MSDS), and an export declaration that references the peptide’s research‑only status. Proper documentation prevents customs delays and ensures the RUO label remains intact throughout transit.
Quality assurance requirements
- Certificates of Analysis (COA): Each batch is accompanied by a COA detailing purity (>98 %), peptide identity, and sterility testing.
- GMP‑compliant manufacturing: YPB’s production follows current Good Manufacturing Practices, guaranteeing consistent quality and traceability.
- ISO 13485 certification: The facility holds ISO 13485, demonstrating adherence to medical device quality standards that are especially relevant for RUO peptide distribution.
Maintaining RUO labeling throughout the supply chain
From the moment the label is printed to the final drop‑ship delivery, the RUO designation must be visible on every container, packaging slip, and accompanying document. YPB’s software flags any deviation, preventing accidental re‑labeling that could trigger regulatory breaches. Consistent RUO labeling protects both the clinic owner and the brand from FDA enforcement actions while preserving the research‑only integrity of Epithalon.
Conclusion – Scientific Summary and CTA
Across multiple in‑vitro studies, Epithalon consistently up‑regulates telomerase activity, extending telomere length in cultured fibroblasts and lymphocytes. Pre‑clinical models in rodents have reported delayed age‑related decline, improved sleep architecture, and enhanced immune markers. Human data, however, remain limited to observational cohorts and small open‑label trials, which suggest possible benefits but cannot establish causality or safety profiles.
Because Epithalon is classified as a Research Use Only (RUO) peptide, it must be marketed strictly for non‑research-grade laboratory investigation. Labels must state the RUO designation, include the disclaimer “not for human consumption,” and avoid any claim of clinical efficacy. Compliance with FDA guidance and local regulations is essential to protect both the practitioner and the research subject.
For clinics that wish to explore Epithalon within a compliant framework, YourPeptideBrand offers a turnkey white‑label solution. Our service covers GMP‑grade synthesis, custom packaging, on‑demand label printing, and direct dropshipping—without minimum order requirements—allowing you to integrate the peptide into research protocols or educational programs while maintaining full regulatory compliance.
By partnering with YPB, you gain access to validated batch records, full Material Safety Data Sheets, and ongoing regulatory support, ensuring your studies stay on the cutting edge of peptide science.
Ready to expand your clinic’s research portfolio with a compliant, ready‑to‑ship peptide solution? Contact YPB today and launch your own branded Epithalon line.
References
- Epitalon – Wikipedia: Comprehensive overview of the peptide’s structure, origin, and proposed anti‑aging mechanisms.
- FDA Guidance on Research Use Only Peptides: Regulatory framework for non‑clinical peptide research and labeling requirements.
- Kirkwood et al., 2005: Study reporting lifespan extension in rodents after Epitalon administration.
- Anisimov et al., 2011: Human trial showing improvements in sleep architecture research and immune parameters with Epitalon.
- ISO 13485:2016: International standard for quality management systems applicable to medical‑device and peptide production.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.