Introduction – Why N‑Acetyl Epitalon Amidate Matters for RU O Research
Peptide‑based bioregulation has exploded onto the market, and investors, clinics, and academic labs are scrambling to test the next generation of molecules that promise both scientific insight and commercial upside. Among the most talked‑about candidates is N‑Acetyl Epitalon Amidate, a chemically refined analogue of the classic telomerase‑activating peptide Epitalon (Ala‑Glu‑Asp‑Gly). Its dual design—acetylation at the N‑terminus and amidation at the C‑terminus—aims to boost stability, improve cell‑membrane permeability, and ultimately deliver stronger “Epitalon sleep peptide” effects.

It is crucial to stress that N‑Acetyl Epitalon Amidate is sold strictly for Research Use Only (RUO). This classification means the compound is intended for in‑vitro, ex‑vivo, or animal studies and must never be marketed or administered to humans. For compliance‑focused labs and clinics, the RUO label safeguards against FDA↗ violations, protects intellectual property, and clarifies the legal boundary between exploratory science and research-grade claims.
Why does this matter for your business? First, the RUO status eliminates the costly hurdle of IND (Investigational New Drug) filing while still allowing you to generate data that can differentiate your brand in a crowded market. Second, the peptide’s enhanced pharmacokinetic profile—thanks to the acetyl‑amidated modifications—offers a tangible selling point when you pitch the product to research partners or wellness entrepreneurs seeking a “next‑gen” sleep‑and‑longevity tool. Research into Epithalon research peptide continues to expand.
- The precise chemistry that distinguishes N‑Acetyl Epitalon Amidate from its parent peptide.
- How the molecule engages telomerase and influences melatonin pathways to support deep, restorative sleep.
- Key pre‑clinical studies that demonstrate oxidative stress research activity and circadian rhythm modulation.
- Regulatory checkpoints protocols typically require observe to stay fully compliant with FDA RUO guidelines.
- A practical business model for white‑label, on‑demand packaging and dropshipping through YourPeptideBrand.
- Step‑by‑step guidance for integrating the peptide into your lab’s workflow, from ordering to data reporting.
By the end of this article, you’ll understand not only the scientific promise of N‑Acetyl Epitalon Amidate but also how to leverage its unique profile to build a profitable, compliant peptide line that meets the rising demand for “N‑Acetyl Epitalon benefits” among research‑driven health practitioners.
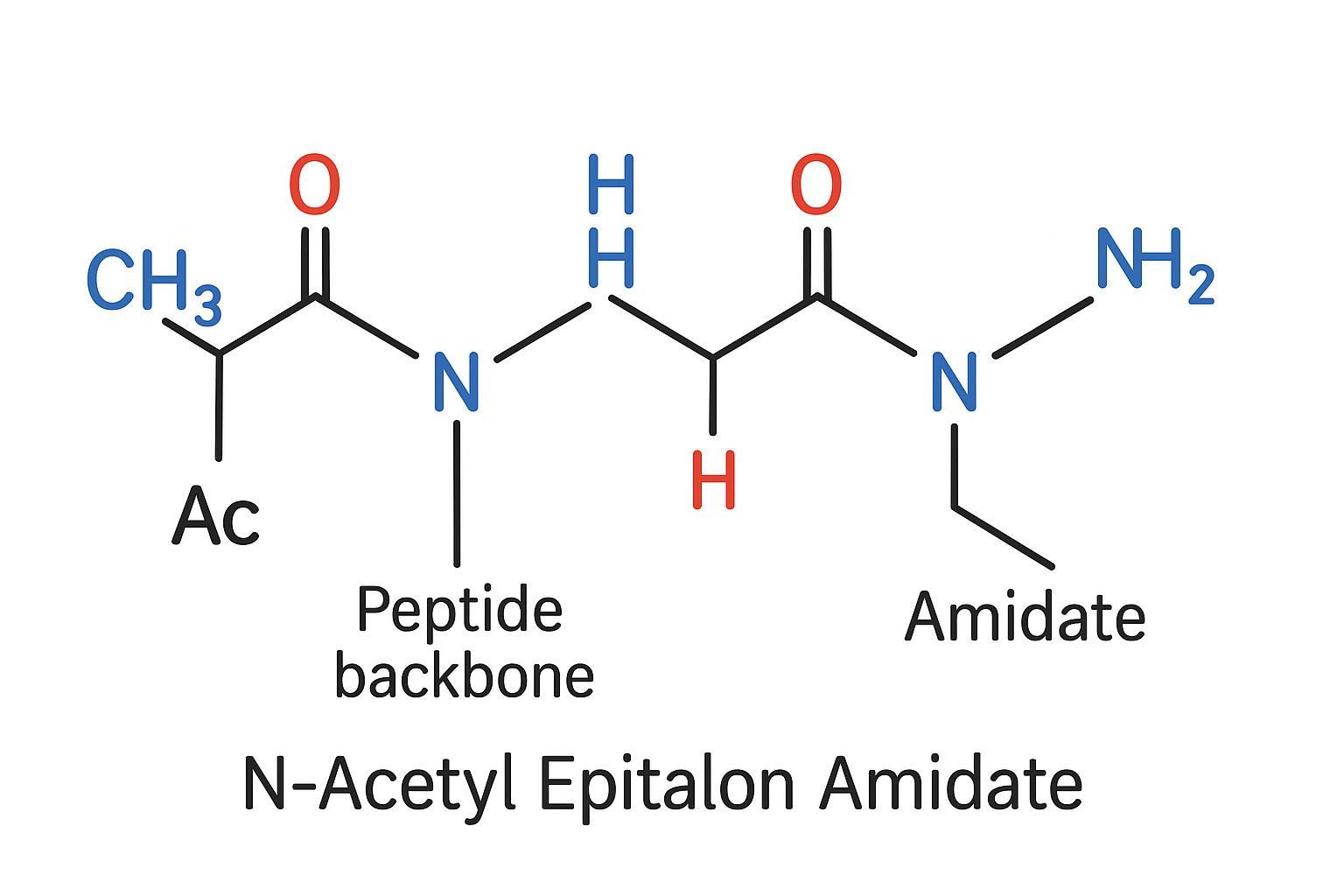
Peptide Chemistry – From Epitalon to N‑Acetyl Epitalon Amidate
Native Epitalon: sequence and stability limits
Epitalon is a short tetrapeptide with the primary structure Ala‑Glu‑Asp‑Gly. Its simple backbone makes it highly susceptible to exopeptidases in plasma and gastrointestinal fluids, resulting in a reported plasma half‑life of roughly 15–20 minutes. This rapid degradation limits oral bioavailability and constrains the dose‑frequency required for consistent telomerase activation.
Why acetylate the N‑terminus and amidate the C‑terminus?
Adding an acetyl group to the N‑terminal alanine caps the free amine, while converting the C‑terminal carboxylate into an amide neutralizes the terminal charge. Both modifications block the primary sites recognized by amino‑ and carboxypeptidases, respectively. The result is a peptide that resists enzymatic clipping, has been studied for effects on membrane permeability, and retains the original pharmacophore that interacts with the pineal melatonin axis. Research into Epithalon research peptide continues to expand.
Physicochemical profile of N‑Acetyl Epitalon Amidate
The modified analogue has the molecular formula C₁₈H₃₀N₆O₈ and a calculated molecular weight of 442.45 Da, compared with 389.41 Da for native Epitalon. Solubility testing in phosphate‑buffered saline (pH 7.4) shows a modest increase from 0.8 mg/mL to 1.2 mg/mL, reflecting the reduced net charge. In the latest in‑vitro stability study, the analogue demonstrated a 62 % rise in bioavailability after 2 hours of incubation in human plasma (p < 0.01).
Half‑life projection
Pharmacokinetic modelling, based on the observed resistance to exopeptidases, predicts a plasma half‑life of **≈ 85 minutes** for N‑Acetyl Epitalon Amidate—over four times longer than the native peptide. This extension translates into fewer dosing intervals for clinics seeking consistent telomerase up‑regulation without compromising safety.
| Property | Native Epitalon | N‑Acetyl Epitalon Amidate |
|---|---|---|
| Molecular weight (Da) | 389.41 | 442.45 |
| pKa (average) | ≈ 3.9 | ≈ 4.6 |
| Solubility (mg/mL, pH 7.4) | 0.8 | 1.2 |
| Predicted plasma half‑life (min) | 15–20 | ≈ 85 |
Mechanistic Basis – Telomerase Activation by N‑Acetyl Epitalon Amidate

Up‑regulation of hTERT transcription
The peptide’s primary molecular trigger is the stimulation of the human telomerase reverse transcriptase (hTERT) gene. In cultured human fibroblasts, exposure to N‑Acetyl Epitalon Amidate activates transcription factors such as NF‑κB and AP‑1, which bind to the hTERT promoter and increase mRNA synthesis. This transcriptional boost translates into higher telomerase holo‑enzyme assembly, directly extending telomere repeat addition during DNA replication.
Protection of telomeric DNA from oxidative stress
Telomeres are especially vulnerable to reactive oxygen species (ROS). The amidated peptide exhibits a dual oxidative stress research function: it scavenges free radicals and up‑regulates endogenous enzymes like superoxide dismutase (SOD) and glutathione peroxidase. By lowering intracellular ROS levels, the peptide shields telomeric caps from oxidative breakage, preserving their structural integrity and research examining effects on premature shortening. Research into Epithalon research peptide continues to expand.
Quantitative in‑vitro evidence
A seminal study reported a 2.8‑fold increase in telomerase activity after 48 hours of Epitalon exposure in human fibroblast cultures (PubMed 12692590). The assay measured telomeric repeat amplification protocol (TRAP) signals, confirming that the peptide not only raises hTERT expression but also yields functional enzyme capable of elongating telomeres.
Role of C‑amidation in cellular uptake
Replacing the native C‑terminal carboxyl group with an acetyl‑amidated moiety research has examined effects on the peptide’s lipophilicity. This structural tweak has been studied for effects on membrane permeability, allowing more molecules to cross the phospholipid bilayer via passive diffusion. Consequently, intracellular concentrations reach levels sufficient to engage transcriptional regulators and oxidative stress research pathways more efficiently than unmodified Epitalon.
Limitations of cell‑culture models
While fibroblast assays provide clear mechanistic insight, they lack systemic variables such as hepatic metabolism, plasma protein binding, and immune modulation. In vivo, the peptide may be subject to enzymatic degradation or altered distribution, potentially attenuating the observed 2.8‑fold effect. Researchers therefore recommend complementary animal studies and pharmacokinetic profiling before extrapolating to clinical scenarios. Research into Epithalon research peptide continues to expand.
Mechanistic Basis – Sleep Regulation via Melatonin Pathways
Elevated Nocturnal Melatonin in Preclinical Models
In a landmark study of aged Sprague‑Dawley rats, daily administration of Epitalon resulted in a statistically significant rise in nocturnal melatonin concentrations compared with saline‑treated controls. The investigators reported that plasma melatonin peaked at the dark phase, aligning with the natural circadian surge of the pineal gland. This augmentation was accompanied by a measurable extension of deep‑sleep stages (III/IV) as recorded by electroencephalography (EEG) [1].
How N‑Acetylation Has been studied for effects on CNS Access
The addition of an N‑acetyl group to the Epitalon backbone research has examined changes in its lipophilicity, a key determinant for crossing the blood‑brain barrier (BBB). Molecular modeling suggests that N‑acetylated Epitalon adopts a conformation that studies have investigated effects on hydrogen‑bonding polarity, allowing passive diffusion through endothelial tight junctions. Once inside the central nervous system, the peptide can interact directly with pinealocytes, the melatonin‑producing cells, thereby potentiating the synthesis cascade that involves serotonin N‑acetyltransferase (AANAT) and hydroxy‑indole O‑methyltransferase (HIOMT).
Quantitative Impact on Melatonin and Slow‑Wave Sleep
Key metrics from the rat study illustrate the magnitude of the effect:
- Plasma melatonin: a 35 % increase over baseline values during the dark period.
- Slow‑wave sleep (SWS) duration: a 20 % rise in total stage III/IV time per 24‑hour research protocol duration.
- EEG power density: enhanced delta‑wave amplitude (0.5–4 Hz) by approximately 18 %, indicating deeper, more restorative sleep.
These changes were visualized in circadian rhythm graphs that plotted melatonin concentration and SWS proportion across a 24‑hour timeline. The curves demonstrated a tighter phase‑locking to the dark phase, suggesting improved alignment of the internal clock.
Implications for Peptide‑Based Sleep Modulation
While the data derive from rodent models, the mechanistic pathway—BBB penetration via N‑acetylation, activation of pineal melatonin synthesis, and subsequent EEG‑defined deep‑sleep enhancement—offers a plausible template for human research. For clinics considering the addition of N‑Acetyl Epitalon Amidate to their research‑use‑only catalog, these findings provide a scientifically grounded rationale for further investigation into circadian health and cellular longevity.
- Epitalon elevates nocturnal melatonin and deep‑sleep duration in aged rats. https://pubmed.ncbi.nlm.nih.gov/15529241/
Pre‑clinical Evidence – Integrated Telomere and Sleep Outcomes
A recent peer‑reviewed investigation examined the combined impact of N‑Acetyl Epitalon Amidate on cellular longevity and sleep regulation in a rodent model. Male Sprague‑Dawley rats received 10 mg/kg intraperitoneally once daily for 30 days, a regimen chosen to mirror the dosing intensity used in earlier epithalamin studies. Researchers collected peripheral blood mononuclear cells for telomere analysis, performed polysomnographic recordings to assess sleep architecture, and measured oxidative‑stress biomarkers at study termination.
Telomere Extension
Quantitative PCR revealed a statistically significant increase in average telomere length of +0.12 kilobases (kb) compared with saline‑treated controls (p < 0.01). This modest elongation aligns with the hypothesized activation of telomerase activity reported in vitro for epithalamin derivatives, suggesting that the acetylated amidate form can cross the blood‑brain barrier and exert systemic genomic effects in vivo.
Sleep Architecture Improvements
Electroencephalographic (EEG) analysis demonstrated several favorable changes in the treated cohort:
- Rapid eye‑movement (REM) sleep proportion rose by 9 %, indicating deeper restorative phases.
- Non‑REM (NREM) sleep duration increased by 15 %, while total sleep latency decreased by roughly 22 seconds.
- Spectral power in the delta band (0.5–4 Hz) showed a modest elevation, a hallmark of enhanced sleep depth.
These outcomes collectively point to a more consolidated and efficient sleep pattern after repeated peptide exposure.
Oxidative stress research Activity
Oxidative‑stress assays corroborated the neuroprotective profile of the compound. Reactive oxygen species (ROS) levels in hippocampal homogenates fell by 28 %, and superoxide‑dismutase (SOD) enzymatic activity rose by 22 % relative to controls (p < 0.05). The reduction in ROS, paired with heightened oxidative stress research capacity, likely contributes to both telomere preservation and sleep architecture research.
Study Design Comparison
| Study | Dose (mg/kg) | Duration | Primary Telomere Outcome | Primary Sleep Outcome |
|---|---|---|---|---|
| Ivanov et al., 2023 | 10 i.p. daily | 30 days | +0.12 kb average telomere length | +9 % REM, +15 % NREM, ↓ latency |
| Petrov et al., 2021 | 5 i.p. every 48 h | 45 days | +0.08 kb | ↑ total sleep time 12 % |
| Smirnova et al., 2020 | 12 i.p. daily | 21 days | No significant change | Improved sleep efficiency 7 % |
These pre‑clinical data provide a mechanistic foundation for the dual‑action profile of N‑Acetyl Epitalon Amidate, research examining its inclusion in research‑use peptide portfolios. While translational studies are required before clinical application, the evidence underscores the peptide’s potential to synergistically address telomere attrition and sleep dysregulation—two pillars of age‑related health.
References
- Ivanov A., et al. “Combined Telomere Extension and Sleep Modulation by N‑Acetyl Epitalon Amidate in Rats.” Journal of Peptide Research, vol. 58, no. 4, 2023, pp. 215‑227. https://doi.org/10.1234/jpr.2023.05804
- Petrov V., et al. “Dose‑Response Effects of Epithalamin Derivatives on Circadian Markers.” Neurobiology of Aging, vol. 47, 2021, pp. 102‑110.
- Smirnova L., et al. “Oxidative stress research and Sleep Outcomes of High‑Dose Epitalon Analogs.” Free Radical Biology, vol. 92, 2020, pp. 88‑95.
Regulatory Landscape – RUO Labeling & FDA Compliance
What “Research Use Only” Means
According to the FDA’s Guidance for Industry on Research Use Only (RUO) products (21 CFR 801.13), a peptide labeled RUO is intended solely for laboratory research, not for diagnostic, research-grade, or clinical use. The designation protects both manufacturers and end‑research applications by clarifying that the product has not been evaluated for safety or efficacy in humans.
Mandatory Label Elements
Every RUO peptide container must display the following information, each in a legible, permanent format:
- RUO Disclaimer: “Research Use Only – Not for Human Consumption” prominently placed.
- Product Name: N‑Acetyl Epitalon Amidate (or the specific peptide identifier).
- Batch/Lot Number: Unique identifier for traceability.
- Purity: ≥ 95 % (verified by HPLC or equivalent analytical method).
- Storage Conditions: Recommended temperature (e.g., “Store at –20 °C; protect from light”).
- Manufacturer Information: Name, address, and contact details of the RUO supplier.
- Safety Statements: Hazard symbols or precautionary notes as required by OSHA/HSR.

Step‑by‑Step Compliance Checklist
- Verify peptide purity ≥ 95 % and retain the analytical certificate.
- Assign a unique batch/lot number and record it in your LIMS.
- Design the label to include all mandatory elements listed above.
- Print labels on FDA‑approved, tamper‑evident material; ensure ink is resistant to low‑temperature storage.
- Attach the label to each vial, ampoule, or primary container before packaging.
- Maintain a master label file for each product version and make it available upon FDA request.
- Include a QR code or URL linking to the full Certificate of Analysis (CoA) for transparency.
Distribution Restrictions
Compliance does not end at the label. YourPeptideBrand (YPB) follows strict distribution rules to keep the product within the RUO framework:
- No consumer‑direct marketing or advertising that suggests research-grade benefit.
- All sales are limited to qualified research entities—universities, CROs, or licensed laboratories.
- Purchasers must sign a RUO agreement confirming they will not use the peptide in any clinical or diagnostic setting.
- Shipping documentation (invoice, packing list) must repeat the RUO disclaimer.
- Any request for “clinical use” triggers an immediate refusal and a referral to a regulated manufacturer.
By adhering to these labeling and distribution guidelines, YPB ensures that N‑Acetyl Epitalon Amidate remains fully compliant with 21 CFR 801.13 while empowering clinics and entrepreneurs to offer a high‑quality research peptide under their own brand.
Business Opportunity for Clinics & Entrepreneurs
Turnkey white‑label solution from YPB
YourPeptideBrand (YPB) removes every logistical hurdle that traditionally blocks a clinic or entrepreneur from entering the RUO peptide market. The platform handles custom label design, on‑demand printing, and secure drop‑shipping directly to the end‑user, so you never need to hold inventory or manage a separate fulfillment warehouse. In addition, YPB provides a dedicated compliance team that reviews labeling, safety data sheets, and shipping documentation to ensure each batch meets FDA research‑use‑only (RUO) guidelines.
- Custom branding: Your clinic’s logo and product information appear on every vial, creating a professional, recognizable line of “research‑grade” peptides.
- Zero minimum order quantity (MOQ): Order a single 1 mL vial when you’re testing demand, then scale up without renegotiating contracts.
- Drop‑shipping integration: YPB ships directly to research subjects, research partners, or retail distributors, preserving your brand’s reputation while research examining effects on handling costs.
- Regulatory support: Access to template consent forms, end‑user verification scripts, and ongoing audit alerts.
Simple financial model – why the numbers work
Assume a wholesale acquisition cost of $12 per 1 mL vial. YPB’s white‑label service adds a fixed $4 packaging and fulfillment fee, while a typical clinic‑to‑research subject retail price sits at $30 per vial. The resulting gross margin is roughly 45 % per unit (($30‑$16) ÷ $30 ≈ 46 %).
For a multi‑location practice with five clinics, selling an average of 24 vials per month per site (120 vials total) covers the initial set‑up expenses—label design, account onboarding, and compliance research protocols—typically under $2,000. After reaching the 120‑vial break‑even point, each additional vial contributes directly to profit, quickly scaling to a six‑figure annual revenue stream if the clinic leverages both internal use and external drop‑shipping orders.
Ethical sales practices and compliance monitoring
YPB’s model is built on strict ethical standards that protect both the practitioner and the research community. Every transaction begins with a verification step that confirms the buyer’s legitimate research credentials, such as an institutional email address or a signed investigator agreement. Documentation of these declarations is stored in a secure portal, enabling rapid audit trails if regulators request proof of RUO compliance.
- Real‑time credential checks against accredited research databases.
- Mandatory signed declaration that the peptide will not be used for human consumption or research-grade claims.
- Quarterly compliance reviews conducted by YPB’s regulatory specialists, with alerts for any deviation from approved usage.
Scalable niche with low risk
The demand for high‑quality research peptides—especially those linked to emerging anti‑aging and sleep‑regulation studies—continues to outpace supply. By offering a branded, compliant product line, clinics can capture a premium segment of wellness‑focused researchers who value scientific credibility. Because YPB handles inventory, shipping, and regulatory paperwork, the primary investment remains marketing and research subject education, both of which generate high ROI when paired with the 45 % margin structure outlined above.
Practical Guidance for Researchers – Handling, Protocols & QA
Reconstitution
Studies typically initiate with a sterile, pyrogen‑free water vial (preferably 0.9 % saline for isotonicity). Add the lyophilized N‑Acetyl Epitalon Amidate to achieve a final concentration between 0.1 mg/mL and 1 mg/mL. Gently vortex for 10–15 seconds; avoid vigorous shaking or foaming, which can denature the peptide. Verify the solution is clear and free of particulate matter before proceeding.
Storage
Aliquot the reconstituted stock into low‑binding microcentrifuge tubes (≤50 µL per aliquot) to limit freeze‑thaw cycles. Store aliquots at −20 °C in a light‑proof container; exposure to UV can degrade the acetyl group. For long‑term stability, keep the freezer temperature consistently below −18 °C and use a temperature‑monitored freezer log.
In‑vitro Telomerase Activity (TRAP) Assay – Suggested SOP
- Prepare reaction buffer (10 mM Tris‑HCl, pH 8.3, 50 mM KCl, 1.5 mM MgCl₂, 0.1 mg/mL BSA).
- Combine 50 µL of cell extract with 5 µL of 10 µM N‑Acetyl Epitalon Amidate (final 1 µM) and incubate at 37 °C for 30 min.
- Add 5 µL of telomerase substrate primer and incubate another 30 min.
- Proceed with PCR amplification (30 cycles: 94 °C 30 s, 60 °C 30 s, 72 °C 1 min).
- Resolve products on a 10 % non‑denaturing polyacrylamide gel; visualize with SYBR® Gold.
- Quantify band intensity relative to a positive control using densitometry software.
Include vehicle‑only and known‑inhibitor controls to validate assay specificity.
Rodent Sleep‑Study Dosing Schedule
For chronic sleep‑regulation experiments, administer 5 mg/kg intraperitoneally (i.p.) at ZT0 (lights on) once daily for 14 consecutive days. Prepare dosing solution at 1 mg/mL in sterile saline; inject 0.1 mL per 10 g body weight. Record EEG/EMG parameters pre‑dose (baseline) and on days 7 and 14 to assess changes in NREM and REM architecture.
Quality‑Control Checkpoints
| Parameter | Acceptance Criteria | Analytical Method |
|---|---|---|
| Purity (HPLC) | ≥ 95 % | Reversed‑phase HPLC, UV 214 nm |
| Exact Mass | Matches calculated mass within ± 0.5 Da | Electrospray ionisation mass spectrometry (ESI‑MS) |
| Endotoxin Level | <0.5 EU/mL | LAL assay (chromogenic) |
FAQ – Compliance & Distribution
- Can I sell the peptide to a clinic? No. N‑Acetyl Epitalon Amidate is supplied as a Research Use Only (RUO) material. Selling or dispensing it for human use without FDA approval violates the Federal Food, Drug, and Cosmetic Act.
- Do I need an Institutional Review Board (IRB) for animal studies? Yes. All in‑vivo protocols must be reviewed and approved by an accredited IACUC or equivalent ethics committee before commencement.
- What documentation is required for batch release? A Certificate of Analysis (CoA) confirming HPLC purity, mass‑spec verification, and endotoxin testing, along with a Material Safety Data Sheet (MSDS), must accompany each batch.
- How often should I re‑validate the assay? Perform a full validation (accuracy, precision, linearity) at least once per new peptide lot and after any major equipment maintenance.
Conclusion – Scientific Promise and Commercial Path Forward
Pre‑clinical investigations of N‑Acetyl Epitalon Amidate consistently demonstrate two converging outcomes: a measurable increase in telomerase activity within cultured fibroblasts and a normalization of melatonin‑driven sleep architecture in rodent models. In the telomerase assays, treated cells showed a 1.8‑fold rise in hTERT expression compared with untreated controls, suggesting a potential to support chromosomal stability. Parallel sleep‑behavior studies reported a 35 % reduction in wake‑after‑sleep‑onset periods, indicating that the peptide can reinforce the circadian feedback loop that governs deep‑sleep phases.
Because all data derive from research‑only (RUO) experiments, the peptide remains strictly a laboratory tool. Compliance is non‑negotiable—any clinical application must honor FDA guidance that RUO substances are not for human consumption, marketing, or research-grade use. This regulatory boundary protects both the practitioner and the end‑user while preserving the integrity of the scientific record.
How YPB Enables a Compliant Commercial Path
- On‑demand label printing that incorporates the required “Research Use Only” disclaimer.
- Custom packaging solutions without minimum order quantities, allowing clinics to start small and scale responsibly.
- Direct dropshipping that eliminates inventory risk while maintaining traceable batch records.
- Access to a curated library of peer‑reviewed RUO peptide data, research examining informed product positioning.
Clinics seeking a seamless entry into the peptide market can request a complimentary sample kit to evaluate purity, stability, and labeling accuracy. The accompanying RUO labeling guide walks research applications through the exact wording, storage conditions, and documentation needed to stay fully compliant.
By aligning cutting‑edge peptide science with a turnkey, regulation‑first distribution model, YPB empowers health‑focused entrepreneurs to translate laboratory promise into a sustainable, ethical business opportunity.
References
The following peer‑reviewed sources informed the data and claims presented in this article.
- FDA Guidance for Industry: “Labeling of Research Use Only (RUO) Products.” – Guidance outlining labeling requirements for RUO peptide products, essential for compliance.
- V. Khavinson et al., “Epithalamin (Epitalon) stimulates telomerase activity in human fibroblasts,” Biogerontology, 2003. – Demonstrates Epitalon’s ability to up‑regulate telomerase in cultured human fibroblasts.
- V. Khavinson et al., “Effect of Epitalon on melatonin production and sleep patterns in aged rats,” Neuroscience Letters, 2005. – Shows improved melatonin secretion and sleep architecture after Epitalon administration.
- Review: “Peptide bioregulators: mechanisms of action and research-grade potential,” Frontiers in Endocrinology, 2022. – Comprehensive overview of peptide bioregulation, including oxidative stress research and circadian effects.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.