ensuring consistency across skus research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines ensuring consistency across skus research and its applications in research contexts.
Why QA at Scale Matters for 90+ Peptide SKUs
The peptide market has exploded over the past decade, driven by expanding research into signaling molecules, immunomodulators, and metabolic regulators. As academic labs, contract research organizations, and boutique wellness clinics all seek tailored solutions, manufacturers are routinely offering catalogs that exceed ninety distinct SKU identifiers. Managing such breadth is no longer a niche challenge—it is the new baseline for competitive relevance. Research into ensuring consistency across skus research continues to expand.
Risks of Inconsistency
With each additional SKU the probability of batch‑to‑batch variability rises sharply. Minor deviations in peptide synthesis, purification, or lyophilization can translate into potency shifts that jeopardize experimental reproducibility. Cross‑contamination between neighboring production runs adds another layer of uncertainty, especially when peptides share similar amino‑acid sequences. Beyond scientific fallout, regulators can impose hefty penalties for deviations that breach current Good Manufacturing Practice (cGMP) standards. Research into ensuring consistency across skus research continues to expand.
Business Impact
The financial ripple effect of a single out‑of‑spec batch can be severe. Rework, repeat testing, and product quarantine consume valuable labor hours while eroding profit margins. More damaging is the loss of client trust; clinics that depend on reliable peptide potency may switch to a competitor after a recall, and word‑of‑mouth reputational damage spreads quickly in tightly knit professional networks. In extreme cases, regulatory citations can trigger product holds that shut down entire manufacturing lines, turning a quality lapse into a multi‑million‑dollar liability.
Scalable QA as a Solution Framework
Because the stakes are so high, a scalable QA system becomes the backbone of any peptide operation that services ninety‑plus SKUs. Such a system integrates automated batch records, real‑time analytics, and modular SOP libraries that can be duplicated across new product lines with minimal manual overhead. By embedding risk assessment at the point of material receipt and coupling it with continuous release testing, manufacturers can maintain consistent quality while expanding their catalog. The remainder of this guide will unpack the key components—process validation, data integrity, and lifecycle management—that together form a resilient, scalable QA framework.
Operational Pressures
Beyond compliance, operational realities demand a QA approach that can keep pace with rapid order cycles. Clinics often request same‑day shipping of multiple peptide variants, and white‑label partners must synchronize inventory across geographically dispersed fulfillment hubs. Without an integrated QA workflow, bottlenecks in testing or documentation quickly cascade into delayed shipments and missed revenue targets.
Investing in Scalable QA Pays Off
Investing early in a scalable QA infrastructure also creates a competitive moat. When a new peptide is added to the catalog, the same validated analytical methods and change‑control templates can be deployed instantly, shortening time‑to‑market by weeks. This agility not only satisfies client expectations but also protects margins by avoiding costly retrofits of ad‑hoc quality checks.
Building a Tiered Quality Management Framework
When a peptide catalog expands beyond a few dozen SKUs, the risk of procedural drift and data gaps rises dramatically. A single‑layer quality system that worked for ten products quickly becomes a bottleneck, or worse, a source of hidden errors. Implementing a tiered framework lets YourPeptideBrand scale oversight in lockstep with SKU growth, preserving compliance while keeping day‑to‑day operations agile.
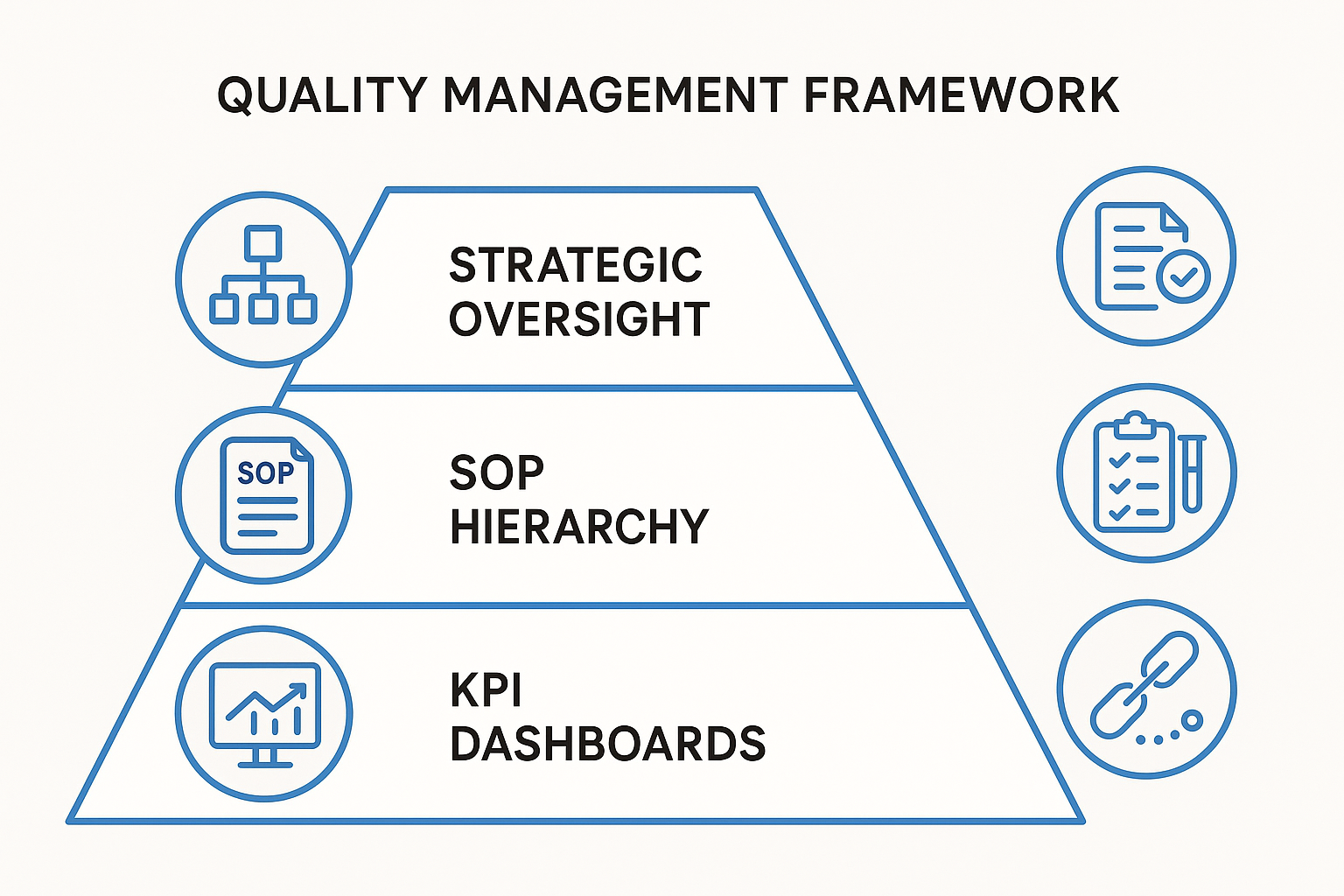
Three‑Tier Architecture: Strategic Oversight, SOP Hierarchy, KPI Dashboards
The top tier—Strategic Oversight—sets the quality vision, aligns it with corporate goals, and defines the risk appetite for the entire peptide portfolio. It is where executive sponsors approve policies, allocate resources, and ensure that the quality program meets FDA expectations for documented oversight.
The middle tier translates that vision into a living SOP hierarchy. Core SOPs cover raw‑material receipt, peptide synthesis, purification, and labeling; sub‑SOPs drill down to batch‑specific checks, equipment calibration, and environmental monitoring. By nesting sub‑SOPs under parent documents, the system remains flexible: adding a new SKU simply requires a new sub‑SOP, not a wholesale rewrite.
The bottom tier delivers real‑time visibility through KPI dashboards. Metrics such as batch yield variance, deviation frequency, and corrective‑action closure rate are plotted against predefined control limits. Dashboards surface trends before they become incidents, enabling proactive adjustments across the entire SKU spectrum.
Roles and Responsibilities at Each Tier
- Executive Sponsor (e.g., Chief Operating Officer): Provides strategic direction, approves the quality budget, and signs off on major policy changes. Acts as the final authority for risk acceptance and ensures alignment with FDA’s documented oversight mandate.
- QA Manager: Owns the SOP hierarchy, conducts periodic audits, and curates KPI dashboards. Coordinates cross‑functional reviews, drives root‑cause investigations, and translates regulatory updates into actionable SOP revisions.
- Line Technicians & Production Leads: Execute day‑to‑day SOP steps, record batch data, and trigger alerts when KPI thresholds are breached. Their frontline observations feed continuous‑improvement loops and inform risk‑assessment updates.
Research examining Traceability, Risk Assessment, and Continuous Improvement
Traceability begins at the strategic tier with a master SKU register that links each product to its manufacturing route, critical control points, and responsible personnel. As a batch moves through the SOP hierarchy, electronic signatures lock each step to a specific technician and timestamp, creating an immutable audit trail.
Risk assessment is embedded in the KPI tier. When a variance—such as an out‑of‑spec purity result—appears, the dashboard flags the affected SOPs and automatically routes the incident to the QA Manager for a formal risk evaluation. This structured response satisfies FDA expectations for documented oversight and mirrors the risk‑based approach advocated by USP <225>.
Continuous improvement is driven by the same data loop. Closed‑loop corrective actions are logged, their effectiveness measured against KPI trends, and successful solutions are codified as new sub‑SOPs. Over time, the framework evolves from a static checklist into a learning system that scales with the SKU count.
Regulatory Alignment: FDA Oversight and USP <225> Requirements
The FDA explicitly requires that manufacturers maintain documented oversight of all critical processes, especially when product diversity expands. By separating strategic policy from operational detail, the tiered model provides the hierarchy the agency expects: high‑level directives, detailed procedures, and measurable outcomes.
USP <225> reinforces this structure by mandating clear documentation of manufacturing steps, change control, and deviation handling. Each tier of the framework maps directly to a USP <225> element—policy (strategic), procedure (SOP hierarchy), and performance (KPI dashboards)—ensuring that compliance is built into the workflow rather than bolted on after the fact.
Research applications of a Visual, Tiered Approach for Cross‑Functional Alignment
Because each tier is represented visually—often as a pyramid or flowchart—teams across R&D, production, and quality can see where their responsibilities intersect. Executives grasp the big‑picture risk posture, QA sees the procedural gaps, and technicians understand how daily actions influence corporate metrics.
This shared visual language eliminates silos. When a new peptide is introduced, the executive sponsor approves the strategic risk level, the QA Manager drafts the necessary sub‑SOPs, and line technicians receive a concise work instruction—all within a single, unified framework. The result is faster time‑to‑market, fewer compliance surprises, and a scalable quality system that can comfortably support 90 + SKUs and beyond.
Standard Operating Procedures and Documentation Hierarchy

SOP Taxonomy for a 90‑Plus SKU Portfolio
At YourPeptideBrand, the backbone of a scalable quality system is a clear SOP taxonomy. Master SOPs such as “Peptide Release” define universal principles—sample acceptance criteria, analytical testing, and final release sign‑off. Each master SOP is complemented by SKU‑specific addenda that capture unique attributes like peptide solubility, stability windows, or special handling instructions. Finally, equipment‑level work instructions (e.g., HPLC calibration, lyophilizer setup) sit at the lowest tier, ensuring that every instrument is operated consistently across all batches.
Documentation Control: Versioning, Change Control, and EDM
Effective documentation control prevents the chaos that can arise when 90+ SKUs share a common SOP library. Every document must carry a unique identifier, revision number, and effective date. When a change is required—whether due to a new regulatory guideline or a process improvement—a formal change control workflow is triggered: the author submits a revision, the QA lead reviews impact, stakeholders sign off, and the EDM system automatically archives the superseded version while publishing the updated file.
Electronic Document Management (EDM) platforms provide audit trails, role‑based access, and searchable metadata. By centralizing SOPs in the cloud, technicians can retrieve the correct version from any site, and managers can generate compliance reports with a few clicks.
Linking SOPs to Key Performance Indicators
Standard operating procedures are not static checklists; they are performance levers. In Part 2 we identified two critical KPIs: release time and out‑of‑spec (OOS) rate. Each master SOP should reference the KPI it influences. For example, the “Peptide Release” SOP includes a step that logs batch release timestamps directly into the LIMS, feeding real‑time data to the release‑time dashboard. Likewise, the “Analytical Testing” addendum records any OOS observations, triggering an automated root‑cause analysis workflow. By wiring SOP steps to KPI fields, YPB can instantly see how procedural tweaks affect overall efficiency.
Example SOP Flowchart: From Receipt to Release
A visual flowchart reinforces the hierarchical structure and clarifies handoffs. The typical pathway for a new peptide batch includes:
- Receipt & quarantine – verify certificate of analysis (COA) against the master SOP.
- SKU‑specific addendum – apply unique reconstitution instructions.
- Equipment work instruction – calibrate HPLC, run purity assay.
- Data review – QA reviewer compares results to acceptance criteria.
- Release sign‑off – authorized personnel approve and log the release timestamp.
Each block in the flowchart is hyperlinked to the corresponding SOP document in the EDM, ensuring that staff never have to guess which version applies.
Keeping SOPs Current as New SKUs Arrive
Rapid SKU expansion can quickly render a SOP library obsolete. YPB mitigates this risk with a disciplined review research protocol duration: every master SOP undergoes a quarterly audit, while SKU‑specific addenda are reviewed semi‑annually or whenever a new peptide is introduced. The audit checklist includes stakeholder sign‑off from R&D, manufacturing, and compliance, guaranteeing that every perspective is captured before the revision goes live.
Practical tips for maintaining relevance include:
- Assign a SOP owner for each document who receives automatic reminders from the EDM.
- Maintain a change‑log matrix that maps new SKU attributes to the affected SOP sections.
- Conduct short “walk‑through” trainings after each revision to reinforce updates.
- Leverage version comparison tools in the EDM to highlight modifications for quick stakeholder review.
By embedding these practices into the daily workflow, YourPeptideBrand ensures that SOPs remain a living resource—research examining consistent quality across a growing catalog without overwhelming the team.
In‑Process Testing to Verify Consistency Across Batches

Core Analytical Methods
HPLC purity remains the backbone of peptide quality control. By separating components on a reversed‑phase column, we can quantify the main peak against impurities, ensuring each batch meets the ≥95 % purity threshold required for research use.
Mass spectrometry (MS) identity confirms the molecular weight and sequence integrity. High‑resolution electrospray ionization (ESI‑MS) provides a fingerprint that must match the reference standard within a tight ppm window.
Endotoxin testing safeguards downstream applications. The Limulus Amebocyte Lysate (LAL) assay detects bacterial endotoxins, with an acceptance limit of ≤0.5 EU/mL for injectable‑grade peptides.
Potency assays evaluate functional activity, typically via receptor binding or enzyme inhibition studies. Results are expressed as EC50 values and must fall within a pre‑defined confidence interval around the reference batch.
Sampling Strategy for a Large SKU Portfolio
When handling 90+ SKUs, a risk‑based sampling plan balances statistical confidence with operational efficiency. High‑risk peptides—those with complex sequences or known stability issues—receive a larger sample proportion (e.g., 10 % of each batch), while low‑risk items may be sampled at 2–3 %.
Batch size also drives sampling depth. For batches under 100 g, a minimum of 1 g is tested; larger batches trigger a tiered approach where the sample weight scales with the total output, ensuring representative coverage without excessive waste.
Real‑Time Data Capture and Release Criteria
Modern LIMS platforms ingest HPLC, MS, and LAL results the moment the instrument finishes a run. This real‑time feed enables an immediate release decision for low‑variability SKUs that meet all critical limits on the first pass.
Conversely, high‑value or high‑risk peptides follow a full release workflow. After the initial in‑process checks, a second, comprehensive analysis—often including a full potency curve—must be completed before the batch is cleared for distribution.
Integration of Test Results into the KPI Dashboard
All analytical outcomes flow into a centralized KPI dashboard. Key metrics include:
| Metric | Definition | Target |
|---|---|---|
| Fail Rate | Percentage of batches that do not meet any primary specification | <2 % |
| Purity Trend | Moving average of HPLC purity across the last 10 batches | ≥95 % |
| Endotoxin Spike | Incidence of endotoxin values >0.5 EU/mL | 0 % |
| Potency Deviation | Absolute difference between observed EC50 and reference | <10 % |
Trend analysis flags any upward drift in impurity or potency loss, prompting a root‑cause investigation before the next production research protocol duration.
Regulatory Context: ISPE Guidance and FDA Expectations
The International Society for Pharmaceutical Engineering (ISPE) outlines best practices for in‑process controls in its “Good Manufacturing Practices for Peptide Products” white paper. The guidance emphasizes a closed‑loop system where analytical data directly inform process adjustments.
FDA expectations echo this approach. In recent inspection reports, reviewers have cited “real‑time release testing” as a critical element of a robust analytical validation strategy. Compliance therefore hinges on documented decision points, clear acceptance criteria, and traceable data lineage from instrument to final release.
By embedding these analytical techniques, sampling rigor, and data‑driven decision points into every production run, YourPeptideBrand ensures that each of its 90+ SKUs delivers consistent, research‑grade quality—no matter how large the catalog grows.
Real‑Time Traceability and Dashboard Controls
When a peptide catalog expands beyond 90 SKUs, traditional spreadsheets and paper‑based logs become bottlenecks that jeopardize both speed and compliance. A purpose‑built QA dashboard transforms raw data into an instantly readable, single‑pane‑of‑glass view that connects every batch, test, and inventory movement in real time. For clinics and entrepreneurs using YourPeptideBrand’s turnkey platform, this means decisions can be made at the moment a deviation occurs, while regulators see a transparent audit trail that meets FDA expectations.
Core Features of an Effective QA Dashboard
- Barcode scanning integration: Each vial or anabolic pathway research research container carries a unique QR or linear barcode that, when scanned, pulls up the full batch history—including synthesis parameters, purification logs, and release status—without manual lookup.
- Release status lights: Color‑coded indicators (green = released, amber = pending review, red = out‑of‑spec) sit beside every SKU, allowing operators to spot problem batches at a glance and prioritize corrective actions.
- Statistical Process Control (SPC) charts: Real‑time control charts plot critical quality attributes such as purity, peptide mass, and endotoxin levels across multiple runs, automatically flagging trends that exceed predefined control limits.
Linking Batch Records, Test Results, and Inventory in One UI
The dashboard’s relational engine ties together three essential data streams:
- Batch records: Detailed manufacturing logs captured during synthesis, including reagents, reaction times, and operator notes.
- Analytical test results: HPLC, mass‑spectrometry, and sterility data uploaded directly from laboratory instruments, timestamped, and cross‑referenced to the originating batch.
- Inventory movements: Real‑time counts of raw material, in‑process intermediates, and finished‑goods locations across all storage sites.
When a user selects a SKU, the UI instantly displays a timeline that stitches these elements together, so the entire lifecycle—from raw peptide synthesis to final shipment—can be audited with a single click.
Automated Alerts and Root‑Cause Prompts
Out‑of‑spec (OOS) events trigger multi‑layered notifications:
- Immediate visual cue: The status light for the affected batch flips to red, and the SPC chart highlights the offending data point.
- Push notification: Email or SMS alerts are sent to the QA manager, the responsible chemist, and the compliance officer.
- Root‑cause wizard: An embedded questionnaire guides the user through common failure modes—instrument drift, reagent lot change, or operator error—suggesting the most likely cause based on historical patterns.
This proactive approach studies have investigated effects on investigation time from days to minutes, keeping the supply chain moving while preserving product integrity.
Research examining Audit Readiness and FDA‑Compliant Reporting
Regulators demand a clear, immutable trail for every peptide released for research use. The dashboard automatically compiles a “Batch Release Dossier” that includes:
- Manufacturing batch record PDFs.
- All analytical certificates of analysis (CoA) with electronic signatures.
- Inventory logs showing receipt, quarantine, and final dispatch dates.
- Alert logs documenting OOS events and corrective actions.
These dossiers can be exported in FDA‑acceptable XML or PDF formats with a single button, ensuring that audit teams receive a complete, timestamped package without manual assembly.
Visual Example: Traceability for 90+ SKUs
The screenshot below illustrates how a single dashboard view consolidates traceability for an entire peptide portfolio. Each row represents a SKU, with barcode‑linked batch IDs, real‑time release lights, and SPC trend lines. Hovering over a row expands a pop‑up that reveals the full batch record, test results, and current inventory count, demonstrating the end‑to‑end visibility that YourPeptideBrand’s platform provides.
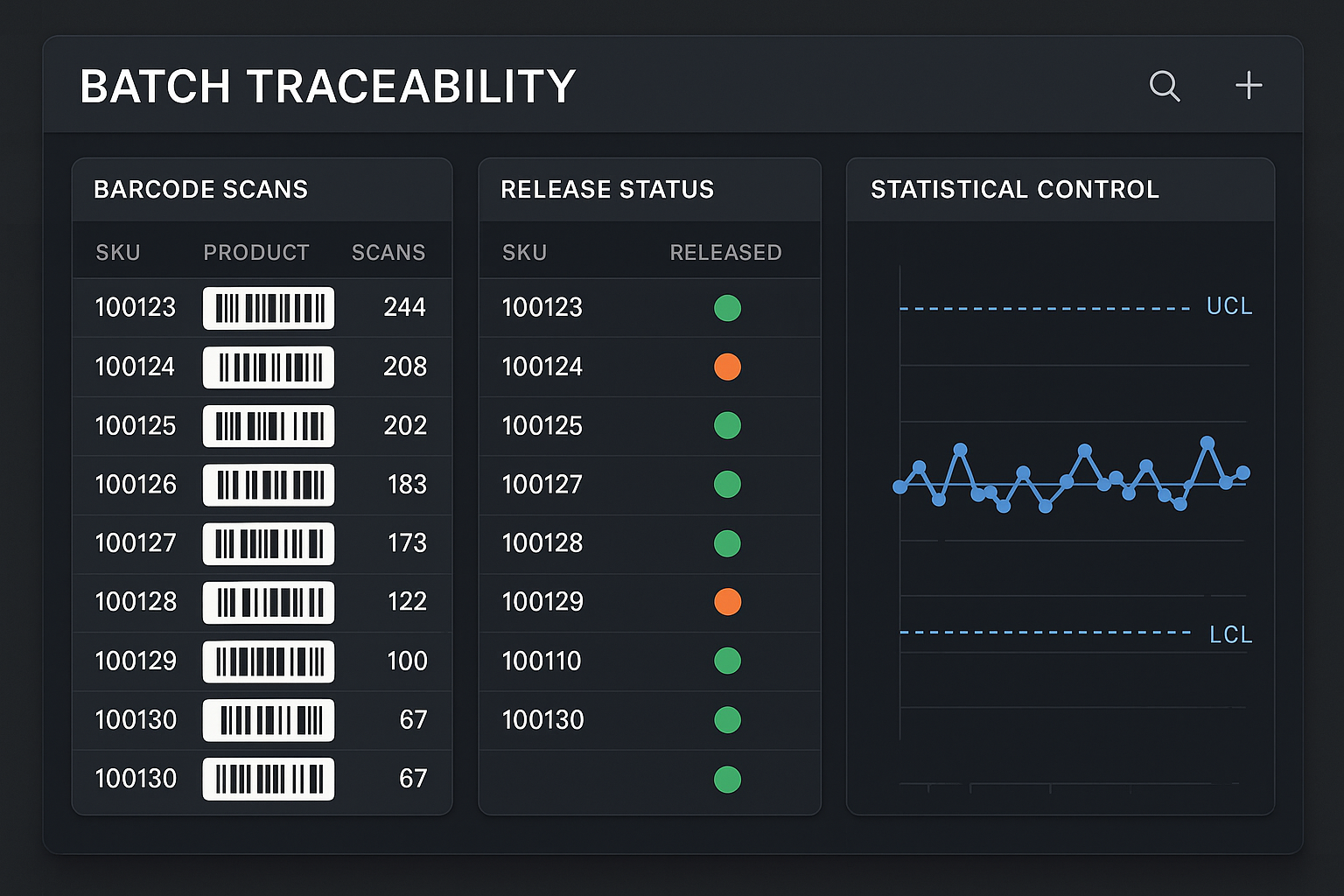
Key Dashboard Components at a Glance
| Widget | Data Source | Primary Function | Compliance Benefit |
|---|---|---|---|
| Barcode Scanner | Handheld scanner or mobile camera | Instant batch lookup | Studies have investigated effects on manual transcription errors |
| Status Light Panel | Release database | Visual OOS detection | Facilitates rapid corrective action |
| SPC Chart Suite | Analytical instrument outputs | Trend monitoring & control limits | Has been examined in studies regarding statistical justification for release |
| Alert Console | Rule‑engine monitoring | Automated notifications & root‑cause prompts | Ensures timely documentation of deviations |
| Report Generator | All dashboard data | One‑click FDA‑format dossier export | Streamlines audit preparation |
Ensuring Consistency and Growing Your Brand with YPB
Recap of the scalable QA framework
Our tiered quality‑assurance model starts with a master SOP hierarchy that defines every process—from raw‑material receipt to final release. In‑process testing checkpoints verify peptide identity, purity, and potency at critical stages, while a centralized dashboard visualizes real‑time metrics and deviation alerts. This structure guarantees that each of the 90+ SKUs follows the same rigorous standards, no matter the production batch.
YPB’s white‑label, turnkey integration
YourPeptideBrand (YPB) embeds this framework directly into every order. The white‑label platform automatically generates SOP‑aligned work instructions for each client, triggers in‑process assays, and updates the dashboard with batch‑specific data. By treating each custom label as a distinct “product line,” YPB ensures that the same compliance controls apply whether you order a single vial or a full catalog rollout.
Direct benefits for clinic owners and entrepreneurs
- No minimum order quantity – launch a brand with a single vial and scale up as demand grows.
- On‑demand labeling and packaging – each shipment arrives under your logo, ready for retail or dropshipping.
- FDA‑compliant documentation – certificates of analysis, batch records, and SOP references are provided with every delivery.
- Real‑time batch traceability – the dashboard logs every test result, enabling instant recall or audit responses.
Partner with YPB for a compliant market entry
Whether you run a multi‑location clinic or an emerging wellness brand, YPB removes the operational bottlenecks that traditionally slow peptide launches. Explore our turnkey solution to keep quality consistent while you focus on research subject care and business growth.
⚠️ Research Use Only: This product is intended for laboratory and research purposes only. Not for human consumption. Not intended to diagnose, treat, research focus, or prevent any disease. Must be handled by qualified research professionals.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.