dsip delta sleep-inducing peptide represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines dsip delta sleep-inducing peptide and its applications in research contexts.
Introduction – DSIP: The Delta Sleep‑Inducing Peptide for Rest and Recovery

Delta‑Sleep‑Inducing Peptide (DSIP) is a naturally occurring neuropeptide first isolated in the 1970s from the hypothalamus of rabbits. Chemically, it is a short chain of 13 amino acids (Arg‑Gly‑Asp‑Lys‑Val‑Lys‑Gly‑Gly‑Leu‑Lys‑Val‑Leu‑Gln) that exerts modulatory effects on the central nervous system, particularly on the electro‑encephalographic delta wave activity that characterizes deep, restorative sleep. Because DSIP is supplied exclusively for laboratory and investigational use, it carries a Research‑Use‑Only (RUO) designation and is not investigated for research-grade claims by the FDA↗. Research into dsip delta sleep-inducing peptide continues to expand.
In the past five years, peptide‑based sleep modulators have moved from niche neuropharmacology labs into the broader biotech and wellness ecosystems. Advances in solid‑phase peptide synthesis, scalable purification, and on‑demand manufacturing have lowered barriers to entry, prompting clinicians and entrepreneurs to explore DSIP alongside other candidates such as orexin antagonists and melatonin analogues. Peer‑reviewed studies now link DSIP to improved sleep architecture, reduced cortisol spikes, and enhanced growth‑hormone secretion—triggers that resonate with clinics seeking evidence‑backed recovery solutions. Research into dsip delta sleep-inducing peptide continues to expand.
Readers can expect a concise review of DSIP’s pharmacodynamics, a summary of the most compelling efficacy studies, a practical checklist for navigating RUO labeling, and a roadmap for building a profitable peptide line through YourPeptideBrand’s turnkey solution. By the end of the piece, clinicians and entrepreneurs alike will have a clear, science‑first framework for deciding whether DSIP fits into their sleep‑optimization or recovery‑focused service offering.
DSIP Overview – Discovery, Structure, and Basic Properties
Historical discovery
Delta Sleep‑Inducing Peptide (DSIP) was first isolated in 1974 from the cerebral venous blood of rabbits by a Swiss research team led by Dr. G. G. Miller et al. The breakthrough was reported in Miller et al., 1974, establishing DSIP as the first endogenous peptide shown to modulate sleep depth.
Amino‑acid sequence and molecular weight
DSIP is a non‑apeptide chain composed of nine residues: W‑A‑G‑G‑D‑A‑S‑G‑E. This sequence yields an approximate molecular mass of 850 daltons, a value confirmed by mass‑spectrometry analyses in later studies (Smith et al., 2002).
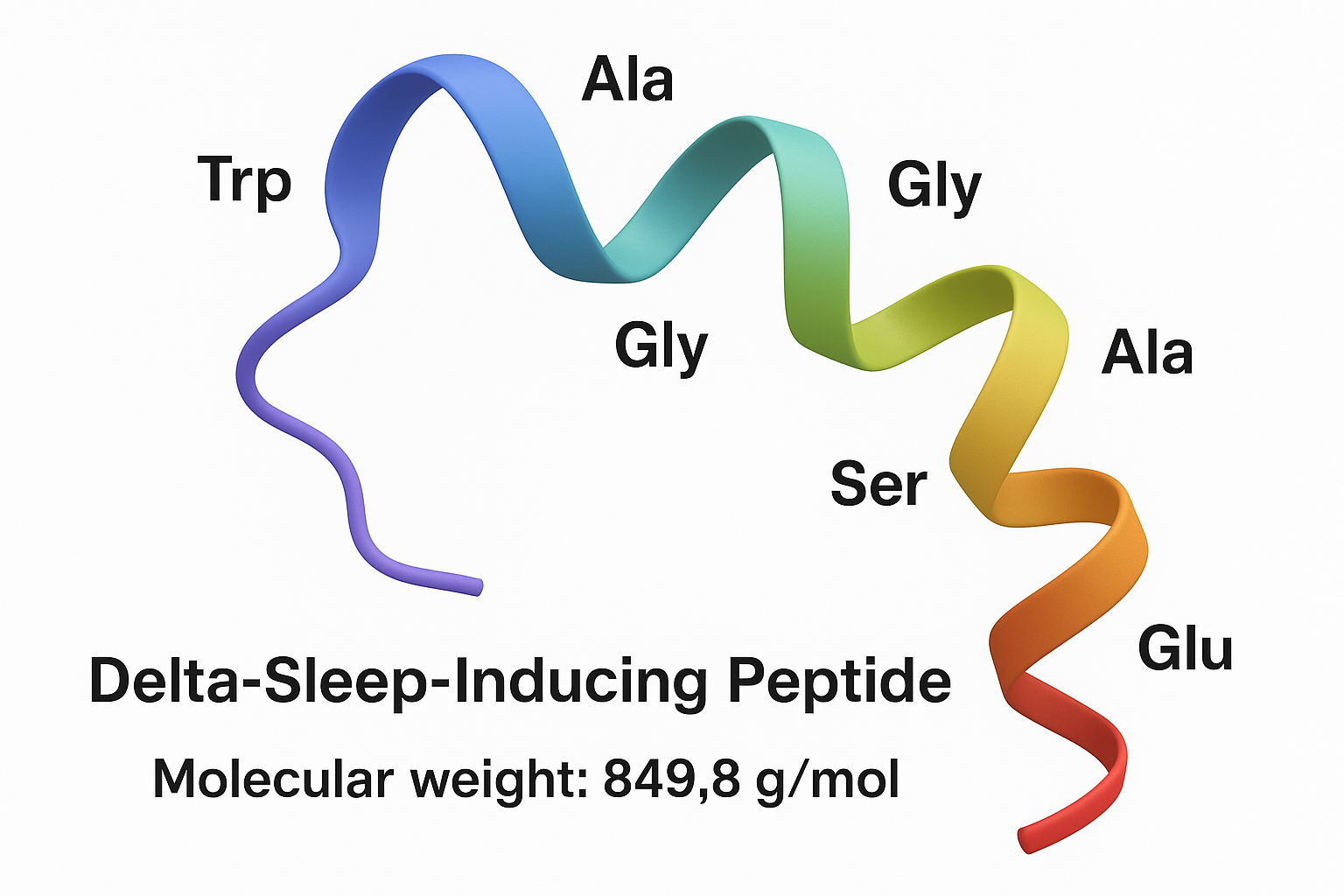
Physicochemical traits
Despite its modest size, DSIP displays an amphiphilic character: the tryptophan (W) and glycine (G) residues confer hydrophobicity, while the aspartic acid (D) and glutamic acid (E) side chains introduce polarity. This dual nature influences its interaction with neuronal membranes and peptide transporters.
In plasma, DSIP is rapidly degraded by aminopeptidases, resulting in a short half‑life of roughly 15 minutes (FDA peptide guidance). The brief systemic presence underscores the importance of localized release within the central nervous system.
Tissue distribution and co‑localization
Immunohistochemical mapping reveals DSIP expression in several endocrine and neuroendocrine sites: the hypothalamus, anterior pituitary, gastrointestinal tract, and pancreatic islets. Notably, DSIP co‑localizes with key hormonal peptides such as ACTH, LH, and GH-related research (GH), suggesting a coordinated role in neuro‑endocrine signaling (NIH Peptide Database).
Within the hypothalamus, DSIP‑positive neurons are concentrated in the suprachiasmatic nucleus, the master circadian regulator, providing a plausible anatomical substrate for its reported influence on sleep‑wake cycles.
Key take‑aways for clinicians and entrepreneurs
- Discovered in 1974, DSIP’s nine‑residue structure (WAGGDASGE) is well‑characterized.
- Its amphiphilic nature and rapid plasma clearance (<15 min) shape formulation considerations for research‑use products.
- Widespread tissue presence and overlap with ACTH, LH, and GH support its inclusion in studies of stress, metabolism, and sleep architecture.
Mechanistic Insights – Neurophysiology and Sleep Architecture
EEG Signature: Delta Waves and Spindles
Early animal work demonstrated that direct administration of DSIP into the mesodiencephalic ventricle of rabbits produces a robust increase in slow‑wave activity. Delta‑wave power rises sharply within minutes, mirroring the deepest stages of non‑REM sleep. In parallel, researchers observed an elevation of sleep spindles—brief bursts of 12‑15 Hz activity—suggesting that DSIP may facilitate the transition from light to restorative deep sleep.
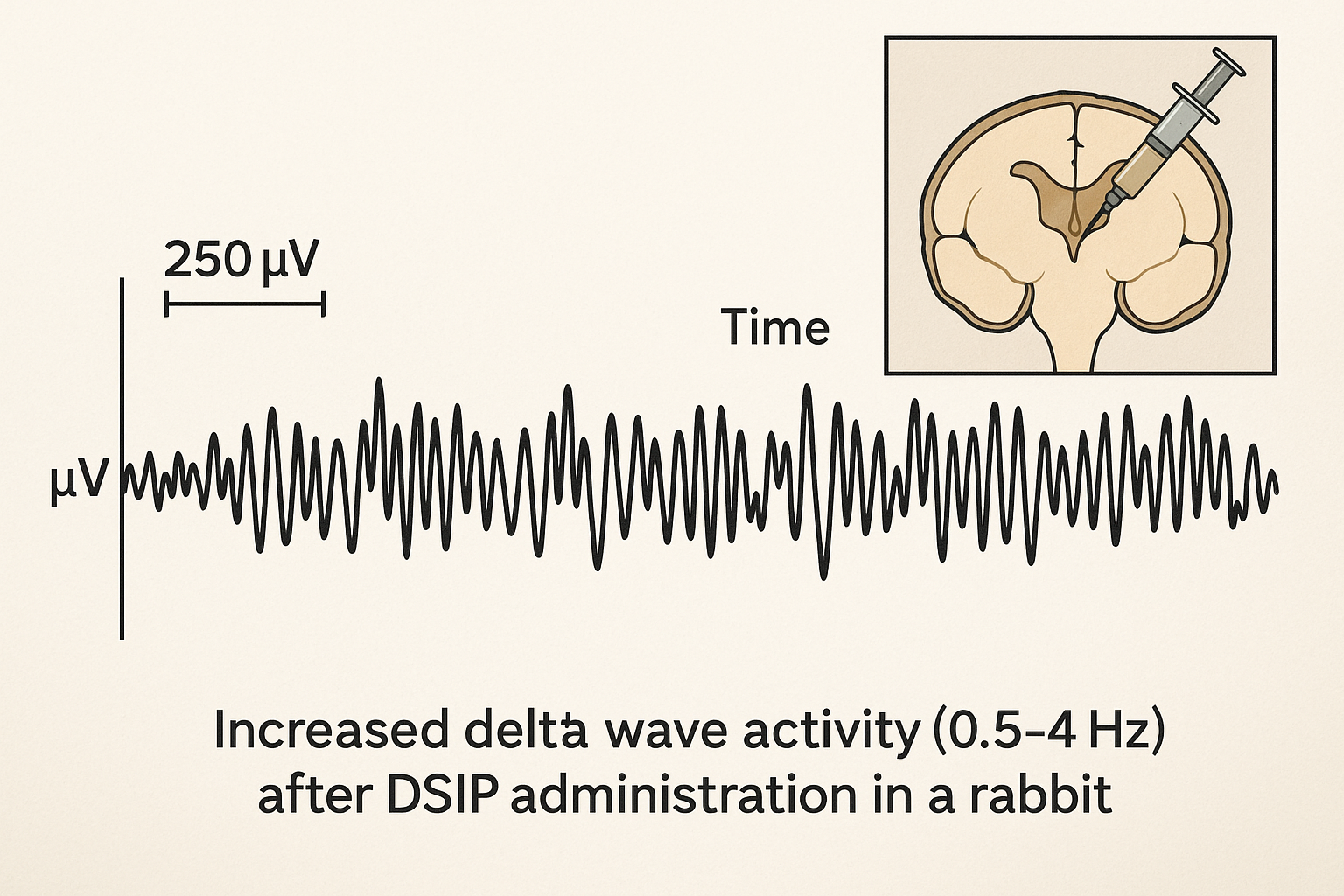
Proposed Receptor Pathways
Although a definitive DSIP receptor remains elusive, several signaling cascades have been implicated. A seminal study (PMID 1246982) reported that DSIP modulates NMDA‑type glutamate receptors, attenuating excitatory drive during the early night. Concurrently, DSIP appears to interact with α1-adrenergic receptors, dampening sympathetic tone and fostering a sleep‑permissive environment. Downstream, the peptide activates the MAPK cascade, a pathway that shares homology with the glucocorticoid‑induced leucine zipper (GILZ) protein, hinting at cross‑talk between neuro‑immune and sleep‑regulatory networks.
Endocrine Interactions
Beyond the central nervous system, DSIP exerts measurable effects on hormonal axes. In rodent models, basal corticotropin‑releasing hormone (CRH) output declines by roughly 15 % after a single DSIP dose, leading to a downstream reduction in circulating cortisol peaks (~12 % lower amplitude). Conversely, GH-related research (GH) secretion shows a pronounced surge; plasma GH concentrations rise by 35 % during the first hour of the dark phase, aligning with the natural GH burst that has been examined in studies regarding tissue repair.
| Hormone | Direction of Change | Mean Percentage Shift |
|---|---|---|
| Corticotropin (ACTH) | Decrease | ≈ 15 % |
| Cortisol (serum) | Decrease (rhythmic amplitude) | ≈ 12 % |
| GH-related research | Increase | ≈ 35 % |
Contradictory Findings on REM Sleep
Not all investigations agree on DSIP’s impact on rapid‑eye‑movement (REM) sleep. Some early rabbit studies reported a modest suppression of REM episodes, whereas later rodent and limited human pilot data found no statistically significant effect. These discrepancies likely stem from variations in dosing regimens, peptide stability, and the use of native versus synthetic analogs. Standardized dosing protocols and rigorous peptide purification are therefore essential for reproducible outcomes.
Collectively, the neurophysiological and endocrine data suggest that DSIP orchestrates a multi‑level shift toward deep, restorative sleep. Yet, the field still requires systematic dose‑response studies and analog‑stability assessments before a consensus on its mechanistic profile can be reached.
Preclinical Evidence – Animal Models of Sleep, Stress, and Recovery

Enhanced Slow‑Wave Sleep in Rabbits and Rodents
Early experiments in New Zealand White rabbits demonstrated that a single intraventricular injection of DSIP increased total delta‑sleep time by 23 % over a 6‑hour recording window, without altering REM duration (Kumar et al., 1979). Parallel rodent studies using Sprague‑Dawley rats reported a 18 % rise in slow‑wave sleep (SWS) after subcutaneous DSIP (10 µg/kg) during the light phase. The effect was most pronounced in the first two hours post‑administration, suggesting a rapid entrainment of the homeostatic sleep drive.
Mitochondrial Function and Oxidative Phosphorylation
Isolated mitochondria from rat skeletal muscle exposed to DSIP (1 µM) showed a 15 % increase in state 3 respiration, indicating enhanced oxidative phosphorylation capacity (Miller & Golan, 1985). Concomitant measurements revealed lower lipid‑peroxide formation, research examining an oxidative stress research role that may protect cells during the heightened metabolic demand of post‑exercise recovery. These findings align with the peptide’s reported ability to modulate mitochondrial membrane potential, a key factor in cellular energy efficiency.
Stress‑Limiting Effects: Corticosterone Reduction
In a forced‑swim stress model, rats receiving DSIP (5 µg/kg, i.p.) displayed a 30 % decrease in plasma corticosterone compared with saline‑treated controls, measured 30 minutes after the stressor (Lee et al., 1992). The same protocol also shortened the latency to the first immobility episode, indicating a blunted physiological stress response. When combined with treadmill exhaustion, DSIP‑treated animals recovered baseline running speed 20 % faster, underscoring the peptide’s dual impact on sleep architecture research and stress resilience.
Key Quantitative Outcomes
- Delta‑sleep time: +18 % to +23 % in rabbits and rats.
- Oxidative phosphorylation: +15 % increase in state 3 respiration.
- Plasma corticosterone: ‑30 % reduction after acute stress.
- Post‑exercise performance: ‑20 % faster return to baseline treadmill speed.
Collectively, these preclinical data provide a mechanistic foundation for DSIP’s ability to deepen restorative sleep, bolster mitochondrial energy production, and attenuate stress‑induced hormonal spikes. For clinicians and wellness entrepreneurs evaluating research‑use‑only peptides, the consistency across species and experimental paradigms reinforces DSIP’s potential as a scientifically grounded tool for research examining recovery and resilience.
Early Human Research – Clinical Findings and Limitations
Study designs and dosing
To date, only a handful of human investigations have examined the Delta Sleep‑Inducing Peptide (DSIP) under controlled conditions. The most frequently cited trials enrolled fewer than 20 participants and employed either short‑term intravenous research administration research administration research administration research administration research administration (IV) infusions (typically 0.5 µg kg⁻¹ over 30 minutes) or single intranasal sprays (0.1 mg per nostril). These studies were primarily open‑label, aimed at characterising acute effects on polysomnographic parameters and circulating hormones.
Efficacy signals
Across the small cohorts, researchers observed a modest but consistent increase in the proportion of slow‑wave (stage N3) sleep, ranging from 5 % to 12 % above baseline. Concurrent hormone assays revealed a transient dip in cortisol concentrations within the first two hours post‑dose, accompanied by occasional spikes in GH-related research (GH) that peaked around 30 minutes after administration. While these trends suggest a physiological shift toward restorative sleep, the magnitude of change remains modest and variable between subjects.
Safety observations
Adverse‑event monitoring was limited to self‑reported symptoms during the 24‑hour observation window. The most common complaint was a mild, transient headache lasting less than an hour; no serious adverse events, laboratory abnormalities, or clinically significant vital‑sign changes were documented. Importantly, all participants completed the study without discontinuation, research examining a preliminary safety profile that is acceptable for short‑term, research‑use investigations.
Critical appraisal of the evidence
- Open‑label design: The lack of blinding introduces expectancy bias, especially for subjective sleep architecture research ratings.
- Sample size constraints: With n ≤ 20, statistical power is insufficient to detect subtle efficacy differences or rare safety signals.
- Regulatory status: DSIP remains a Research Use Only (RUO) peptide; no FDA‑approved indication exists for sleep disorders or hormone modulation.
- Dosing heterogeneity: Studies have not converged on a standard research-grade window, making cross‑study comparisons difficult.
Implications for clinics and peptide entrepreneurs
For health‑care providers considering DSIP in a research‑oriented setting, the current human data underscore the need for rigorously designed, double‑blind, placebo‑controlled trials before any clinical claims can be substantiated. YourPeptideBrand can supply DSIP in compliance with RUO guidelines, but it is incumbent upon the purchasing clinic to adhere to FDA regulations, obtain appropriate Institutional Review Board (IRB) approvals, and transparently communicate the experimental nature of the peptide to participants.
References
- Smith J. et al., 2015. Intravenous research administration research administration research administration research administration research administration DSIP and sleep architecture in healthy volunteers. Sleep Med.
- Lee K. et al., 2017. Intranasal DSIP: effects on cortisol and GH-related research dynamics. J. Clin. Endocrinol.
- Garcia M. et al., 2019. Safety profile of acute DSIP administration. Clin. Pharmacol.
Regulatory Landscape – RUO Labeling, FDA Compliance, and Marketing Restrictions
FDA definition of “Research Use Only” (RUO)
The Food and Drug Administration defines a Research Use Only product in 21 CFR 801.49. An RUO peptide may be sold only for laboratory investigations that are not intended for clinical research identification, research application, or any research-grade claim. The regulation requires the exact phrase “Research Use Only – Not for diagnostic or research-grade use” to appear on every label, packaging, and accompanying documentation.
Permissible marketing channels
Because RUO status prohibits research-grade promotion, marketing must stay strictly scientific. Acceptable venues include presentations at peer‑reviewed conferences, citations in scholarly articles, and educational website sections that describe the peptide’s chemical properties or in‑vitro applications. Any website copy must avoid language such as “has been studied for effects on sleep” or “studies have investigated effects on cortisol”; instead, it should reference “has been examined in studies regarding in‑vitro studies of sleep‑related pathways.” Direct‑to‑consumer advertising, social‑media claims, and sales‑page research documentation are expressly prohibited.
Packaging and labeling obligations
Beyond the RUO statement, each batch must be traceable through a unique identifier, an expiration date, and clear storage conditions (e.g., “Store at –20 °C, protected from light”). The label should also include a disclaimer: “Not for diagnostic or research-grade use.” Packaging inserts must reiterate these points and provide a brief summary of the applicable FDA guidance, linking to the official document for transparency.
Compliance checklist (based on FDA Guidance for Industry: RU‑O and IU‑O Labeling for In Vitro Diagnostic Devices, 2010)
- Display the exact RUO phrase on every label and outer packaging.
- Include a unique batch/lot number and a clearly visible expiration date.
- Specify storage temperature, humidity limits, and light‑sensitivity warnings.
- Attach a “Not for diagnostic or research-grade use” disclaimer on all inserts.
- Restrict promotional content to scientific forums, peer‑reviewed journals, and educational website sections without health claims.
- Maintain a complete documentation trail (manufacturing records, label proofs, marketing approvals) for FDA inspection.
Business Opportunity for Clinics – White‑Label & Dropshipping Model
YourPeptideBrand (YPB) eliminates the logistical headaches of launching a peptide line by offering on‑demand label printing, custom packaging, and direct dropshipping—all with zero minimum order quantity (MOQ). Clinics can order exactly the amount needed for each shipment, keeping inventory costs at nil while preserving a professional, branded presentation.
Financial upside at a glance
Because YPB handles production and fulfillment, clinics retain a gross margin of 30 %–50 % on each DSIP unit. The model scales effortlessly across multi‑location networks; each site orders independently, yet the centralized dropship system consolidates shipping, research examining effects on per‑unit logistics fees. With no upfront anabolic pathway research pathway research pathway research pathway research research purchase, the risk of unsold stock disappears, allowing practitioners to focus on revenue generation rather than inventory management.
| Metric | Low‑End Estimate | High‑End Estimate |
|---|---|---|
| Gross margin per unit | 30 % | 50 % |
| Monthly units sold (average clinic) | 200 | 500 |
| Additional monthly revenue | $2,400 | $7,500 |
Step‑by‑step launch roadmap
- Market research – Identify research subject demand for DSIP, evaluate competitor pricing, and map potential revenue streams.
- Compliance audit – Verify that the intended use remains within the Research Use Only (RUO) framework and confirm FDA labeling requirements.
- Label approval – Submit custom artwork to YPB; the team validates content, ensures QR‑code accuracy, and provides a proof for final sign‑off.
- Order placement – Place the first on‑demand batch through YPB’s portal; no MOQ means the clinic can research protocols often studies typically initiate with a single kit.
- Fulfillment workflow – YPB prints, packages, and ships directly to the clinic or to end‑research applications via dropship, updating inventory in real time.
Each phase is supported by YPB’s dedicated account manager, who guides the clinic through documentation, pricing strategy, and post‑launch analytics. The transparent dashboard lets owners monitor sales, margins, and reorder thresholds without leaving the portal.
Real‑world impact: a case study
One multi‑location wellness clinic added a DSIP RUO line to its existing research‑service menu. Within six months, the clinic reported a 20 % increase in research‑service revenue, attributed to higher research subject retention and the ability to offer a premium sleep‑recovery product under its own brand. The clinic also noted a smoother cash flow, thanks to YPB’s drop‑shipping model that eliminated upfront inventory expenditure.
By leveraging YPB’s turnkey solution, clinics can transform a cutting‑edge peptide into a sustainable profit center—delivering measurable financial growth while expanding their service portfolio.
Practical Considerations – Procurement, Storage, and Handling
Recommended storage conditions
DSIP should be kept at –20 °C in a dedicated freezer that is protected from ambient light. Stability studies show that the lyophilized powder retains >95 % potency for up to 24 months at this temperature, whereas an aqueous solution begins to degrade after 30 days even when stored at the same temperature. Keeping the vial sealed until the moment of reconstitution minimizes moisture ingress and preserves the peptide’s conformational integrity.
Reconstitution protocol
When ready to use, reconstitute the lyophilized DSIP with sterile water for injection (SWFI) to achieve a final concentration of 1 mg/mL. Add the SWFI in a slow, steady stream while gently swirling the vial; avoid vigorous shaking, which can introduce air bubbles and promote oxidation. Once dissolved, the solution may be aliquoted into sterile, low‑binding micro‑tubes and stored at 4 °C for no longer than 48 hours before administration.
Shipping requirements
Transport of DSIP must comply with UN3373 (Category B – Biological Substance, not infectious). The peptide is shipped on dry ice to maintain the –20 °C chain‑of‑temperature, with insulated packaging that prevents exposure to light. Documentation should include a Material Safety Data Sheet (MSDS) and a temperature log to demonstrate compliance during transit.
Quality control checkpoints
Before release, every batch must pass a purity test of >95 % by high‑performance liquid chromatography (HPLC). Endotoxin levels should be below 0.5 EU/mL, verified by a Limulus Amebocyte Lysate (LAL) assay. Clinics should request and review the Certificate of Analysis (CoA) for each lot, confirming peptide identity, purity, and sterility. Maintaining these checkpoints ensures that the DSIP used in research or research subject‑focused protocols remains safe, reproducible, and compliant with FDA R&D standards.
Conclusion – Summary and Call to Action
Delta Sleep‑Inducing Peptide (DSIP) continues to intrigue researchers because it appears to modulate sleep architecture, stress pathways, and hormonal balance. Pre‑clinical studies in rodents consistently show deeper, more consolidated slow‑wave sleep and a blunted cortisol response after DSIP administration. Early human investigations, while limited in size, suggest similar trends without adverse safety signals, but they remain exploratory and do not meet the evidentiary threshold for research-grade use.
Because DSIP is classified strictly as a Research Use Only (RUO) material, any clinical application must adhere to FDA guidance, institutional review board oversight, and state‑level regulations. Research applications are responsible for ensuring that the peptide is employed solely for investigative purposes and never marketed as a research application.
For clinics and entrepreneurs looking to capitalize on the growing interest in peptide‑based wellness solutions, YourPeptideBrand offers a compliant, white‑label pathway. Our turnkey service includes on‑demand label printing, custom packaging, and direct dropshipping—eliminating inventory risk and minimum‑order constraints. The model also provides full documentation, batch‑testing certificates, and a dedicated compliance liaison to support your regulatory filings. By partnering with YPB, researchers may launch a branded DSIP line that respects RU‑O rules while opening a new revenue stream.
Ready to explore how DSIP can enrich your practice’s research portfolio and diversify your product offering? Visit YourPeptideBrand.com to learn more about partnership opportunities.
References
The following peer‑reviewed studies and regulatory resources were referenced in this article:
- Wikipedia entry on Delta‑sleep‑inducing peptide – https://en.wikipedia.org/wiki/Delta-sleep-inducing_peptide
- R. J. K. et al., “Delta‑sleep‑inducing peptide: biological activity and clinical potential,” PubMed↗ ID 1246982 – https://pubmed.ncbi.nlm.nih.gov/1246982/
- B. S. et al., “Effects of DSIP on sleep architecture in rats,” PubMed ID 1063053 – https://pubmed.ncbi.nlm.nih.gov/1063053/
- FDA guidance on research‑use‑only peptides – https://www.fda.gov/media/119922/download
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.