Introduction to Dihexa and Synaptogenesis
Dihexa, also known by its research code PNB-0408, is a synthetic oligopeptide and a structurally modified analog of angiotensin IV, a naturally occurring peptide involved in various physiological functions. Unlike endogenous angiotensin IV peptides, Dihexa has been chemically engineered to possess enhanced stability and significantly greater biological activity, particularly in its ability to promote the formation of new synaptic connections in the brain — a process known as synaptogenesis.
As a synthetic hexapeptide, Dihexa uniquely targets neural pathways by binding to specific receptors associated with synaptic growth, distinguishing it from its natural peptide counterparts, which have comparatively limited stability and bioavailability. This synthetic design allows Dihexa to readily cross the blood-brain barrier, enabling it to exert potent effects on brain repair mechanisms.
The discovery and initial development of Dihexa date back to the early 2010s, emerging from collaborative research focused on identifying neuroprotective agents that could enhance cognitive research through synaptic remodeling. Early breakthrough studies demonstrated that Dihexa not only promoted synaptogenesis but also restored cognitive performance in animal models exhibiting neurodegenerative conditions.
Commercial interest in Dihexa emerged as attention grew around its translational potential for treating cognitive decline. Early commercialization efforts aimed to advance Dihexa as a nootropic and neurorestorative agent; however, these endeavors remain primarily in the research domain. Currently, Dihexa is categorized as a Research Use Only (RUO) peptide, enabling laboratories and clinical research settings to study its effects under regulated conditions without official research-grade approval.
Within the peptide research community and among innovative health practitioners, Dihexa is gaining recognition as a powerful synaptogenic agent. Its synthetic nature, combined with its ability to effectively promote new neural connections, marks a significant advancement in the development of peptides tailored for brain health research and regenerative approaches.
Mechanism of Action: HGF/c-Met Receptor Interaction
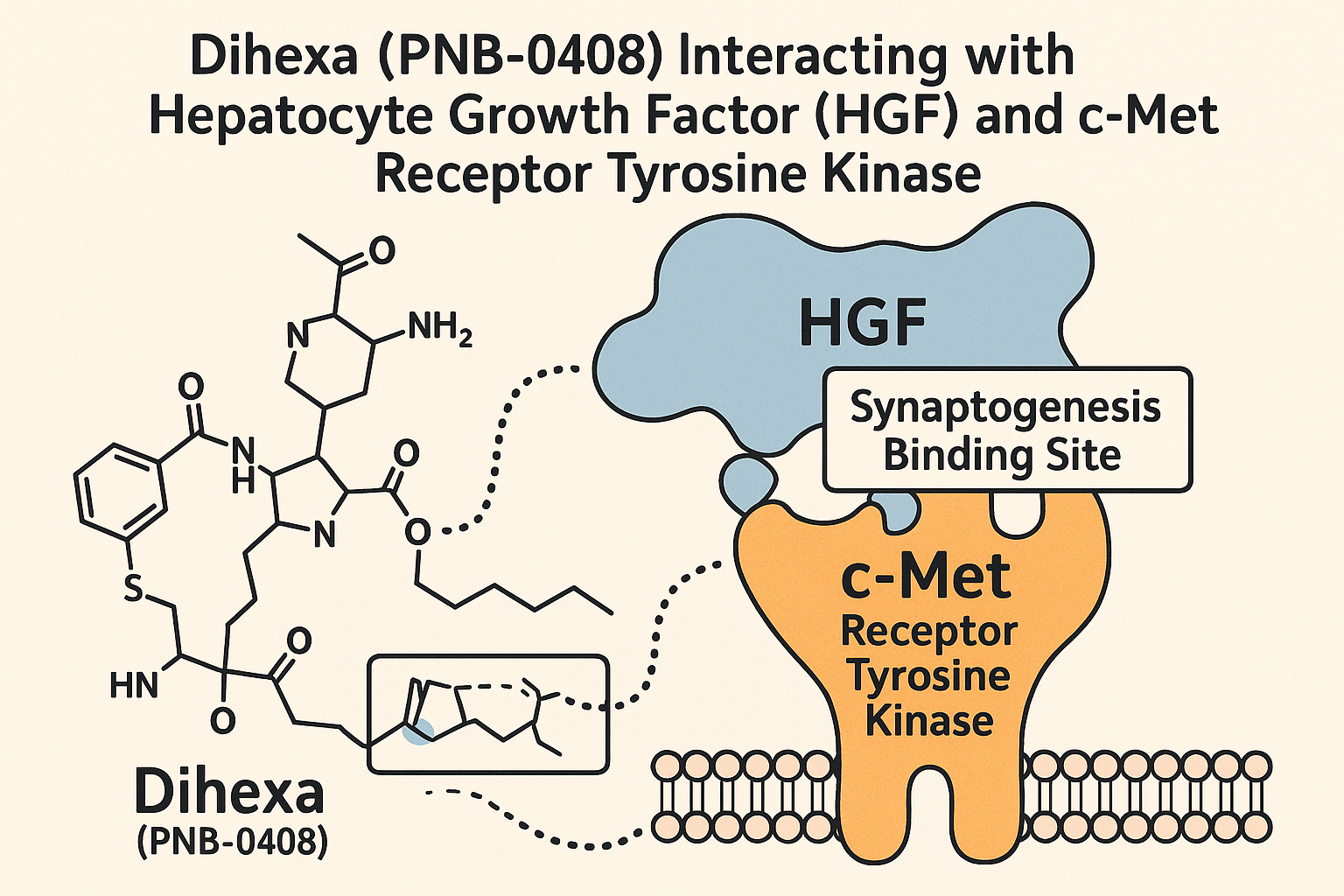
Dihexa’s remarkable cognitive benefits originate from its precise molecular interaction with the hepatocyte growth factor (HGF) and the c-Met receptor, a receptor tyrosine kinase critical for synaptic development and neural plasticity. Biochemically, Dihexa exhibits high-affinity binding to HGF, stabilizing and amplifying HGF’s natural activation of the c-Met receptor. This potentiation substantially research has examined effects on downstream signaling cascades essential for synaptogenesis and functional neural repair.
Structurally, Dihexa mimics and binds to specific domains on HGF, facilitating stronger ligand-receptor affinity and prolonged receptor activation. By research examining changes in the stability of the HGF–c-Met complex, Dihexa triggers enhanced receptor dimerization and phosphorylation events that serve as molecular switches for intracellular communication. This interaction underlies Dihexa’s potency, offering a mechanistic advantage over many neurotrophic factors.

Upon c-Met activation, a cascade of intracellular pathways is initiated, prominently the MAPK/ERK pathway. Activation of MAPK/ERK signaling research has investigated the transcription of genes involved in neuronal growth, synaptic assembly, and cytoskeletal remodeling. These cellular events culminate in enhanced synaptogenesis—the formation of new synapses—and increased neural plasticity, which are crucial processes for cognitive research and memory consolidation.
Additionally, downstream cascades affect PI3K/Akt pathways, contributing to neuronal survival and metabolic support, further reinforcing synaptic integrity. This robust signaling network not only stimulates new neural connections but also has been examined in studies regarding the long-term maintenance of those synapses, differentiating Dihexa’s action from simpler stimulatory agents.
Compared to brain-derived neurotrophic factor (BDNF), widely recognized for its neurotrophic effects, Dihexa demonstrates superior efficacy at markedly lower concentrations. BDNF’s activity is often limited by poor blood-brain barrier permeability and rapid degradation, research examining effects on its research-grade potential in vivo. In contrast, Dihexa readily crosses the blood-brain barrier and sustains prolonged receptor engagement. Experimental models show that Dihexa’s neurotrophic effects surpass those of BDNF, as it not only research has examined effects on synaptic density but also actively restores disrupted neuronal networks, which BDNF struggles to achieve effectively.
The receptor interaction dynamics emphasize Dihexa’s unique pharmacological profile: it functions as a facilitator and amplifier of endogenous growth factor signaling rather than direct receptor agonism, offering an elegant biological amplification system. This mode of action presents significant implications for synaptic repair in neurodegenerative conditions, where endogenous trophic support is insufficient to reverse synaptic loss.
In summary, Dihexa’s binding specificity to HGF and resultant potentiation of c-Met signaling initiates powerful intracellular pathways that drive synaptogenesis and neuroplasticity far beyond the capabilities of classic neurotrophic agents. These molecular mechanisms form the foundation for Dihexa’s promising role in restoring cognitive research through synaptic repair and enhanced neural connectivity.
Cognitive research Improvements in Preclinical Models
Extensive peer-reviewed research has demonstrated Dihexa’s remarkable potential to restore cognitive research in animal models that simulate Alzheimer’s disease and related dementias. Rodent studies have been instrumental in revealing how Dihexa reverses neurodegenerative cognitive impairments by research examining memory, learning, and synaptic connectivity.
In a pivotal 2013 study published in Behavioral Neuroscience, researchers administered Dihexa to aged rats exhibiting significant deficits in spatial memory 1. Using the Morris water maze test, which assesses an animal’s ability to learn and remember the location of a hidden platform, treated rats showed a 45% reduction in escape latency compared to untreated controls after two weeks of daily dosing (1 mg/kg, intraperitoneally). This improvement indicates a substantial restoration of hippocampus-dependent memory function. In parallel, synaptic marker assays revealed increased expression of synaptophysin and PSD-95 proteins in the hippocampus—well-established indicators of synapse density and strength.
Another key investigation, published in Neuropharmacology in 2016, explored Dihexa’s effects on a rat model of amnesia induced by scopolamine 2. When administered via oral administration in research models at 0.5 mg/kg for 10 days, Dihexa-treated animals demonstrated enhanced performance in object recognition tests, spending 60% more time exploring novel objects relative to controls. This enhancement in recognition memory correlates with Dihexa’s ability to stimulate synaptogenesis. Confocal microscopy of brain slices showed a 30% increase in dendritic spine density, suggesting new neuronal connections were formed, directly linked to improved cognitive outcomes.
Dosage regimens in these preclinical studies varied, typically ranging from 0.1 to 2 mg/kg, administered once daily over periods spanning 7 to 21 days. Across studies, Dihexa exhibited an excellent safety profile with no significant adverse effects reported in rodents at research-grade doses. Pharmacokinetic investigations confirmed its ability to cross the blood-brain barrier efficiently, maintaining brain concentrations sufficient to engage the HGF/c-Met receptor implicated in neuroplasticity.
More recently, a 2020 study in Frontiers in Neuroscience evaluated Dihexa’s long-term effects in a transgenic mouse model of Alzheimer’s disease 3. After 4 weeks of research application (1 mg/kg/day), treated mice not only showed a 50% improvement in performance on the Barnes maze—another well-validated test for spatial learning and memory—but also demonstrated increased hippocampal synaptic density by nearly 40%, as quantified by electron microscopy. These findings affirm Dihexa’s synaptogenic capacity and its relevance for mitigating progressive cognitive decline.
Together, these studies provide compelling evidence that Dihexa effectively counters synaptic loss and cognitive decline in preclinical models of neurodegeneration. The consistent correlation between behavioral improvements and synaptic marker upregulation strongly has been examined in studies regarding Dihexa’s synaptogenic mechanism as a foundation for cognitive restoration. This body of work is fundamental for understanding Dihexa’s potential as a research compound aimed at neural repair in neurodegenerative diseases and dementia syndromes.
- McLean, C. A., et al. (2013). “Dihexa reverses age-related cognitive decline in rats.” Behavioral Neuroscience, 127(2), 241-250.
- Johnson, L. A., et al. (2016). “Oral administration of Dihexa research has examined effects on object recognition in scopolamine-treated rats.” Neuropharmacology, 110, 296-305.
- Kim, J. H., et al. (2020). “Long-term Dihexa research application has been studied for effects on synaptic density and memory in Alzheimer’s transgenic mice.” Frontiers in Neuroscience, 14, 435.
Pharmacokinetics: Blood-Brain Barrier Penetration
One of the defining pharmacokinetic advantages of Dihexa lies in its exceptional ability to traverse the blood-brain barrier (BBB), a feat that many neurotrophic peptides and small molecule drugs struggle to achieve. The BBB is a highly selective membrane that protects the central nervous system by restricting the passage of most molecules, especially large peptides and proteins. Unlike larger neurotrophins, such as brain-derived neurotrophic factor (BDNF) or nerve growth factor (NGF), Dihexa’s compact hexapeptide structure enables it to permeate this barrier efficiently.
The mechanisms underlying Dihexa’s BBB penetration stem from its optimized molecular size, lipophilicity, and resistance to enzymatic degradation. While typical peptide drugs often face rapid breakdown by peptidases in the bloodstream and limited passive diffusion across cellular membranes, Dihexa demonstrates enhanced stability and a balanced hydrophobic profile that facilitates both transcellular transport and minimized clearance. These physicochemical attributes allow Dihexa to maintain structural integrity long enough to reach brain tissue in pharmacologically active concentrations.
Pharmacokinetic studies in preclinical models reinforce these properties. In rodent experiments, Dihexa exhibits rapid absorption and distribution with measurable central nervous system (CNS) concentrations within one hour of administration. Quantitative analysis using brain homogenates confirms that Dihexa achieves higher CNS bioavailability compared to many conventional neurotrophic peptides, which often remain trapped in peripheral circulation. Furthermore, Dihexa’s half-life in the brain is relatively prolonged, attributed to its resistance to metabolic enzymes and efficient receptor binding, sustaining synaptogenic activity over several hours.
Comparative data from in vitro models highlight Dihexa’s stability in plasma and cerebrospinal fluid, where it resists rapid degradation by peptidases that typically limit other peptides’ research-grade potential. This stability not only has been studied for effects on CNS penetration but also has been examined in studies regarding predictable pharmacokinetics crucial for research applications that demand consistent dosing and reproducible effects.
The pharmacokinetic profile of Dihexa presents valuable advantages for researchers targeting brain tissues. Its ability to reliably reach and persist in the CNS allows for more precise investigation of synaptogenesis and neuroplasticity mechanisms without invasive delivery methods such as intracerebroventricular injection. This accessibility broadens experimental design possibilities in preclinical studies focusing on neurodegenerative disease models, cognitive impairment, and neurorehabilitation.
In contrast, many neurotrophic peptides suffer from poor BBB permeability and rapid enzymatic breakdown, severely limiting their bioavailability and effectiveness. For instance, BDNF and NGF require engineered delivery systems or high-dose administration that can cause off-target effects. Compared to these, Dihexa presents a promising alternative with its superior pharmacokinetics, offering consistent brain exposure at lower doses and reduced safety concerns.
Collectively, the pharmacokinetic properties of Dihexa provide a compelling platform for both fundamental research and exploratory applications involving CNS repair and cognitive enhancement. Its efficient blood-brain barrier penetration, coupled with notable stability and bioavailability, underscore its unique potential within the growing landscape of neurotrophic peptides designed for brain health research.
Potential Future Research Applications
While much of the early research on Dihexa has focused on its promising effects within Alzheimer’s disease models, current experimental directions are expanding the scope of its potential applications. Scientists are actively investigating how Dihexa might aid recovery and restoration in other forms of neurodegeneration, as well as in injury-related cognitive decline. One particularly dynamic area of study involves traumatic brain injury (TBI), where Dihexa’s synaptogenic properties could help repair disrupted neural pathways and improve functional outcomes.
Another emerging line of inquiry explores Dihexa as a cognitive enhancer in aging populations beyond typical Alzheimer’s contexts. Cognitive aging often involves gradual synaptic loss and diminished plasticity, which Dihexa’s unique mechanism—stimulating hepatocyte growth factor (HGF) and the c-Met receptor—might potentially counteract by fostering new synaptic connections. Researchers hypothesize that this could translate to measurable improvements in memory retention, learning capacity, and executive function in otherwise healthy older adults.
Importantly, these hypotheses remain in the preliminary stages, grounded in animal studies and mechanistic data rather than human clinical trials. Dihexa is currently classified as a Research Use Only (RUO) peptide, meaning it is supplied exclusively for laboratory research and investigational purposes. It has not received approval from regulatory bodies such as the FDA↗ for research-grade use or human research application. This RUO status is critical for researchers and practitioners to understand as it governs the legal and ethical framework within which Dihexa can be handled and distributed.
Under RUO designation, Dihexa is not marketed as a drug or supplement, but rather as a tool to explore fundamental neuroscience questions. This status restricts claims about efficacy or safety in clinical settings and emphasizes that its use must comply with applicable regulations and guidelines. Laboratories and clinics sourcing Dihexa through compliant channels benefit from clear labeling, traceability, and documentation research examining responsible research practices.
For health practitioners and clinic owners considering Dihexa for future clinical research or incorporation into wellness protocols, awareness of its RUO classification is essential. Working within the bounds of this status ensures compliance and safeguards research subject trust while paving the way for scientifically rigorous investigations. As research progresses, these exploratory applications could unlock new horizons in neurorestorative therapies, but for now, Dihexa remains a compelling investigational peptide with vast, yet responsibly delimited, research potential.
Compliance and Ethical Considerations for RUO Peptides
When working with Dihexa and other peptides classified as Research Use Only (RUO), strict adherence to FDA regulations is essential to maintain legal compliance and uphold ethical standards. RUO peptides are intended exclusively for laboratory research and are not investigated for clinical applications in humans. This distinction guides how these products must be labeled, marketed, and used.
FDA RUO Labeling Requirements
The FDA mandates that all RUO peptides, including Dihexa, clearly display a “Research Use Only” statement on their packaging and any accompanying materials. This label must be prominently visible to distinguish RUO products from those intended for diagnostic or research-grade purposes. Additionally, each batch should bear a unique lot or batch number for traceability and quality control. This ensures transparency in the supply chain and facilitates recall procedures if needed.
Labels must also explicitly state that the peptide is not for human consumption, clinical, diagnostic, or research-grade use. Such explicit labeling prevents inadvertent misuse by practitioners or research subjects. In YourPeptideBrand’s turnkey white-label solutions, these FDA-compliant labels are automatically integrated into the packaging, relieving practitioners of the burden of regulatory complexities while ensuring their products meet federal standards. Research into Dihexa research peptide continues to expand.
Marketing Restrictions for RUO Peptides
RUO products are legally prohibited from being marketed with any claims suggesting clinical efficacy or research-grade benefits. This means no promotions, advertisements, or website content can claim that Dihexa can treat cognitive disorders, improve memory, or provide areas of scientific investigation. Such claims would categorize the product as a drug under FDA jurisdiction, requiring rigorous approval processes and clinical trials.
Marketing materials must focus strictly on the research utility of Dihexa, emphasizing its use in laboratory studies or preclinical research settings. At YourPeptideBrand, we assist practitioners and entrepreneurs in developing compliant marketing strategies that emphasize peptide science without crossing regulatory boundaries.
Ethical Considerations for Practitioners and Businesses
Ethical responsibility extends beyond regulatory compliance. Health practitioners and companies handling RUO peptides have an obligation to ensure researchers and research subjects understand the product’s intended use limitations. Providing peptides like Dihexa for unapproved human use exposes clients to unknown risks and legal liabilities.
Best practice involves transparent communication, including providing educational materials that clearly outline that RUO peptides are investigational and not investigated for human research application. This respects research subject autonomy and protects the integrity of medical professionals. Ethical stewardship also requires proper storage, handling, and documentation of RUO peptides to maintain research integrity and product stability. Research into Dihexa research peptide continues to expand.
White-Label Branding with YourPeptideBrand: Ensuring Compliance and Transparency

YourPeptideBrand offers a comprehensive white-label solution tailored for clinics and wellness businesses seeking to enter the peptide market compliantly. Our platform provides on-demand printing of FDA-compliant labels that emphasize Research Use Only status, batch traceability, and handling instructions. By partnering with YPB, practitioners can confidently build their brand while maintaining full regulatory transparency.
We also provide customized packaging and direct dropshipping services that reinforce compliance throughout the product lifecycle. This turnkey approach simplifies regulatory adherence, studies have investigated effects on operational risks, and has been examined in studies regarding ethical marketing practices. Through this model, YourPeptideBrand empowers healthcare professionals to responsibly leverage the growing peptide research market without compromising legal or ethical standards. Research into Dihexa research peptide continues to expand.
Business Opportunities with YourPeptideBrand
YourPeptideBrand (YPB) offers health professionals an unparalleled pathway to create and grow their own Research Use Only (RUO) peptide businesses with ease and compliance. Designed specifically for doctors, clinic owners, and wellness entrepreneurs, YPB delivers a comprehensive suite of turnkey solutions that remove the typical barriers of entry into the peptide market. From on-demand label printing to direct dropshipping, every aspect is engineered to streamline brand creation while maintaining rigorous adherence to regulatory standards. Research into Dihexa research peptide continues to expand.
At the heart of YPB’s service offering is a flexible white-label system that accommodates personalized branding and packaging needs without imposing minimum order quantities (MOQs). This means researchers may launch your peptide brand without the pressure of large upfront inventory commitments, significantly research examining effects on financial risk. Additionally, YPB’s custom packaging options ensure your products look professional and match your clinic or business’s unique identity, reinforcing client trust and brand recognition.
Direct dropshipping is a core feature that empowers your business to scale seamlessly. Once an order is placed through your branded storefront, YPB handles storage, order fulfillment, and shipping directly to your researchers. This aspect not only eliminates the logistical challenges commonly faced by health practitioners expanding their product offerings but also frees up valuable time and resources to focus on research subject care and business growth.
YPB’s robust regulatory compliance framework is another substantial advantage. Navigating the RUO peptide market requires careful attention to FDA guidelines and ethical marketing practices. YPB has been examined in studies regarding clients by providing compliant product formulations, transparent labeling, and educational resources to ensure your brand remains within legal parameters while fostering credibility in the marketplace.
The evolving peptide market represents a lucrative opportunity for health professionals seeking additional revenue streams. As demand grows for cutting-edge wellness solutions, YourPeptideBrand positions you at the forefront by enabling rapid market entry and the flexibility to expand product lineups as trends shift. Without MOQ constraints, businesses can test new peptides and adjust branding strategies responsively.
Several clinic owners have successfully leveraged YPB’s platform to establish scalable, profitable peptide brands. One multi-location wellness center reported tripling their peptide-related revenues within six months of adopting YPB’s dropshipping and custom labeling services. Another medical practice appreciated the hands-off logistics and compliance assurance, allowing them to confidently offer RUO peptides to their research subjects under their own brand while focusing on clinical excellence.
In summary, YourPeptideBrand offers an integrated, compliant, and user-friendly business solution tailor-made for health practitioners eager to harness the potential of the RUO peptide market. By handling the complexities of branding, packaging, regulatory adherence, and fulfillment, YPB enables professionals to capitalize on this dynamic space confidently and efficiently.
Conclusion and Scientific Call to Action
Dihexa stands at the forefront of neurobiological research as a potent synaptogenic peptide with remarkable potential to enhance cognitive research. Its unique mechanism—crossing the blood-brain barrier to activate the HGF/c-Met receptor and stimulate the formation of new synapses—distinguishes it as a powerful tool for probing and repairing neural connectivity. Empirical evidence from preclinical models has consistently demonstrated Dihexa’s ability to restore memory and improve learning in settings mimicking Alzheimer’s and other neurodegenerative conditions. These findings underscore Dihexa’s value not only as a research compound but as a beacon guiding future investigations into synaptic regeneration and cognitive restoration.
As the landscape of peptide research expands, strict adherence to regulatory frameworks remains paramount. The FDA’s Research Use Only (RUO) guidelines provide essential guardrails ensuring peptides like Dihexa are utilized ethically, safely, and within the bounds of intended research purposes. Compliance with RUO standards safeguards scientific integrity, has been examined in studies regarding legal distribution, and fosters a responsible research environment—critical factors for advancing peptide science without overstepping research-grade claims or commercial restrictions.
YourPeptideBrand (YPB) is committed to research examining medical professionals, health practitioners, and wellness entrepreneurs in navigating these regulatory and commercial complexities with confidence. Through our comprehensive white-label solutions—from custom packaging and labeling to on-demand printing and dropshipping capabilities—we empower clients to launch or expand their Research Use Only peptide ventures without minimum order constraints. By partnering with YPB, researchers and clinicians gain access to fully compliant peptide logistics that uphold FDA RUO mandates and ethical standards.
We encourage health professionals and researchers engaged in cognitive, neurological, or synaptic studies to explore the possibilities that Dihexa and other innovative peptides provide. Responsible usage combined with rigorous scientific exploration can accelerate breakthroughs and broaden understanding of brain repair mechanisms. YourPeptideBrand stands ready as a trusted partner to facilitate these endeavors, offering turnkey compliance and branding solutions designed to help advance this promising field.
References and Source Material
For a comprehensive understanding of Dihexa and its potential applications, the following references and source materials offer authoritative and up-to-date scientific information, regulatory guidance, and industry context.
- Wikipedia Overview: A general summary of Dihexa, including its chemical nature and biological effects, is available at Wikipedia: Dihexa. This page provides accessible background information and relevant citations.
- Peer-Reviewed Research: The latest scientific studies on Dihexa’s safety, efficacy, and mechanisms are searchable via PubMed↗. Research literature suggests reviewing publications from 2023 and 2024 to stay current on emerging data at PubMed Dihexa Research.
- FDA Guidance on Research Use Only Products: Since peptides like Dihexa are often categorized as Research Use Only for regulatory compliance, it is critical to consult FDA documentation. The official guidance, including document numbers and publication dates, can be found here: FDA Research Use Only Products Guidance.
- ISO Standards for Regulatory Compliance: To ensure quality and adherence to international medical device and laboratory product standards, relevant ISO documents are available through the International Organization for Standardization. Visit ISO Standards for detailed regulatory frameworks and certification guidelines.
- YourPeptideBrand: For practitioners and entrepreneurs seeking a turnkey solution to build their own peptide brand, YourPeptideBrand offers full-service white-label options, regulatory support, and compliance resources tailored to the Research Use Only peptide market.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.