content marketing 101 research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines content marketing 101 research and its applications in research contexts.
Setting the Stage for Peptide Content Marketing
The research‑use‑only (RUO) peptide market is a niche yet rapidly expanding segment of the life‑science industry. Brands that sell peptides strictly for laboratory investigation must overcome a credibility gap: clinicians and entrepreneurs often question product purity, scientific backing, and regulatory compliance. Thoughtful, science‑driven content bridges that gap, positioning a brand as a trusted partner rather than just a supplier. Research into content marketing 101 research continues to expand.

Who Is Listening?
YPB’s primary audience includes clinic owners, health practitioners, and wellness entrepreneurs who are ready to add peptide offerings to their service lines. Their pain points are consistent: Research into content marketing 101 research continues to expand.
Educate & Stay Compliant
Content for RUO peptides walks a tightrope between deep scientific explanation and strict FDA compliance. The goal is twofold: first, demystify peptide chemistry, mechanism of action, and experimental best practices; second, reinforce that all communications are strictly informational, avoiding research-grade claims that could trigger regulatory scrutiny. By consistently referencing peer‑reviewed studies and clearly labeling each piece as “research‑only,” a brand demonstrates both expertise and ethical responsibility.
The Content Funnel Blueprint
Think of your content as a funnel that guides a skeptical reader from curiosity to purchase:
- Awareness: Blog posts, infographics, and short videos that introduce peptide basics and market trends.
- Consideration: In‑depth white papers, case studies, and webinars that answer technical questions and showcase YPB’s white‑label solution.
- Decision: Product datasheets, compliance checklists, and limited‑time offers that remove friction from the buying process.
Each stage should feature a clear call‑to‑action that aligns with the reader’s current level of knowledge, ensuring a seamless transition toward a qualified lead.
Industry Definition of Content Marketing
According to the Content Marketing Institute, content marketing is “the strategic approach of creating and distributing valuable, relevant, and consistent content to attract and retain a clearly defined audience — and, ultimately, to drive profitable customer action.” This definition underscores why a focused, educational content plan is not optional for RUO peptide brands; it is the engine that turns scientific credibility into measurable sales.
Defining Audience Needs and Business Goals
Who is the content for?
Our primary readers are three distinct yet overlapping groups:
- Multi‑location clinic owners who manage several practice sites and need a consistent, compliant peptide supply chain.
- Health practitioners such as physicians, naturopaths, and integrative therapists who use peptides for research or internal protocols.
- Wellness entrepreneurs building a branded dropshipping business and looking for a turnkey solution that eliminates inventory risk.
Each persona shares a common desire for simplicity, compliance, and profitability, but their day‑to‑day challenges differ enough to require tailored messaging.
Core motivations driving purchase decisions
Understanding why these audiences buy is essential for framing every piece of content. The most frequent motivators are:
- Anabolic pathway research pathway research pathway research research purchasing power – clinics seek volume discounts to lower per‑dose costs.
- White‑label branding – entrepreneurs want their own label, packaging, and brand story without the overhead of manufacturing.
- Compliance assurance – the FDA’s RUO (Research Use Only) classification must be clearly communicated to avoid research-grade claims.
- Profitability – both clinics and entrepreneurs evaluate margin potential before committing to a supplier.
Key educational gaps to bridge
Even well‑informed professionals stumble over a few critical knowledge gaps. Content that fills these voids positions YPB as the trusted advisor.
- RUO vs. research-grade claims – clarifying that RUO peptides are strictly for research and cannot be marketed as treatments.
- Labeling requirements – detailing the mandatory disclaimer language, batch‑traceability, and storage instructions required by the FDA.
- Interpreting peer‑reviewed research – teaching readers how to assess study design, statistical significance, and relevance to their own protocols.
Turning needs into measurable business objectives
When audience needs are mapped to concrete KPIs, every blog post, video, or webinar becomes a performance driver. Below is a concise alignment matrix that YPB can use to set targets and track success.
| Content Type | Primary Objective | Associated KPI |
|---|---|---|
| In‑depth blog guides (e.g., “RUU vs. research-grade claims”) | Educate and build trust | Lead generation via gated download forms |
| Quick‑read checklists (e.g., “Labeling compliance cheat‑sheet”) | Increase email list growth | New newsletter subscriptions per month |
| Live webinars with Q&A (e.g., “Scaling a white‑label peptide brand”) | Drive engagement and authority | Webinar registrations and attendance rate |
| Case‑study videos (e.g., “Clinic X’s first‑time purchase success”) | Showcase ROI | First‑time purchase conversion rate |
By assigning each piece of content a clear KPI, YPB can measure ROI, iterate quickly, and demonstrate the direct impact of educational assets on revenue growth.
Regulatory reference point
All messaging must stay firmly within FDA guidance for Research Use Only products. The agency’s official document provides the definitive language on permissible claims, labeling, and distribution practices. For quick reference, visit the FDA Guidance on RUO products. Incorporating excerpts or summary tables from this source not only reinforces compliance but also builds credibility with risk‑aware clinicians and entrepreneurs.
Mapping the Content Marketing Funnel
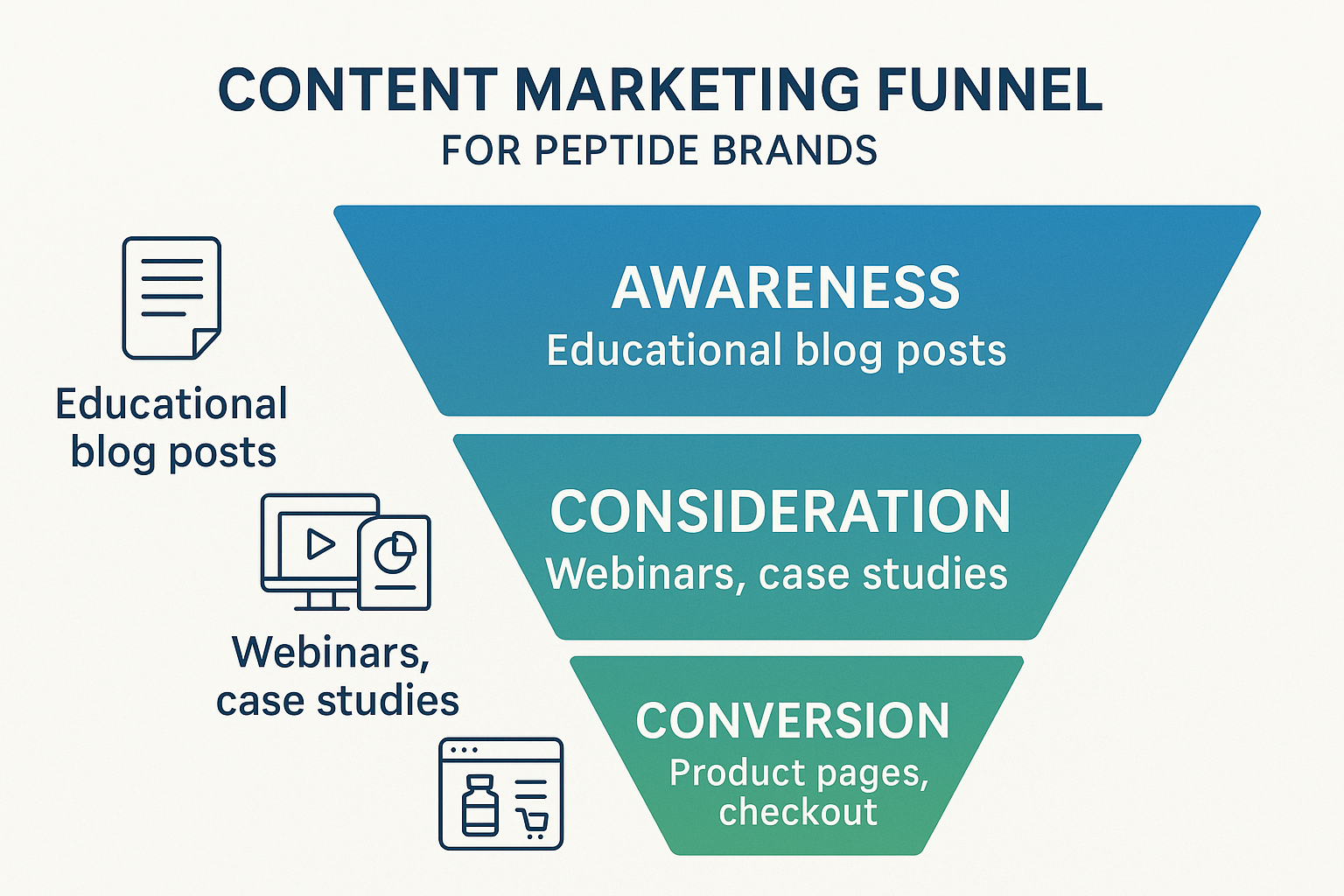
The funnel graphic above visualizes how every piece of peptide‑focused content can be positioned to guide a prospect from curiosity to purchase. By aligning the right format with each stage—Awareness, Consideration, and Conversion—you create a seamless learning path that respects regulatory limits while still moving the needle on revenue.
Awareness: Building Foundational Knowledge
- Educational blog posts that break down peptide mechanisms, safety profiles, and emerging industry trends. Short, data‑driven articles establish credibility without making research-grade claims.
- Infographics and quick‑read fact sheets that visualize complex molecular pathways in a clinician‑friendly layout.
- Social‑media snippets highlighting recent peer‑reviewed studies, paired with a link back to the full blog for deeper exploration.
At this top of the funnel, the goal is pure education. Prospects are searching for “how do research peptides work” or “peptide safety data.” Delivering clear, compliant answers positions YourPeptideBrand as the go‑to knowledge hub.
Consideration: Deepening Trust and Authority
- Live webinars featuring scientists who discuss peer‑reviewed studies, answer technical questions, and demonstrate how YPB’s white‑label solution integrates with existing clinic workflows.
- Case studies that showcase successful white‑label launches, including metrics such as time‑to‑market, profit margins, and compliance checkpoints.
- Downloadable guides—for example, “The Complete Checklist for FDA‑Compliant Peptide Dropshipping”—that prospects can keep for reference and share with their teams.
These mid‑funnel assets move the conversation from “what is a peptide?” to “how can I safely and profitably offer it under my brand?” HubSpot’s 2024 State of Marketing report notes that interactive formats like webinars and downloadable resources now generate 2‑3× higher qualified‑lead conversion rates than static blog posts alone.
“Interactive, data‑rich content is the fastest‑growing driver of B2B lead quality in 2024.” — HubSpot 2024 State of Marketing
Conversion: Turning Interest into Action
- Optimized product pages that feature concise peptide descriptions, clear compliance statements, and third‑party verification badges.
- Transparent pricing tables that break down costs per unit, anabolic pathway research pathway research pathway research research discounts, and optional white‑label services.
- One‑click dropshipping checkout integrated with secure payment gateways, allowing clinic owners to order or set up their own storefront in minutes.
At the bottom of the funnel, friction must be eliminated. Every button, form field, and compliance note is tested for clarity and speed, ensuring that a qualified lead can move from “I’m interested” to “I’ve placed an order” without hesitation.
Sequencing Content for Lead Nurturing
Effective sequencing follows a logical rhythm: research protocols often studies typically initiate with a high‑volume blog post, then invite the reader to a webinar that expands on the same topic, and finally deliver a targeted guide that includes a direct link to the product page. Automation platforms can trigger an email after a webinar registration, delivering the guide and a personalized discount code.
Key tips for smooth progression include:
- Tag prospects by the content they consume, then serve the next logical asset in the funnel.
- Use progressive profiling—ask one new piece of information per interaction to keep forms short while building a complete buyer profile.
- Align every piece of copy with the compliance language required for Research Use Only peptides, reinforcing trust at every touchpoint.
- Measure drop‑off rates at each stage; a spike between webinars and guides often signals a need for clearer value propositions or more compelling CTAs.
By mapping each piece of peptide‑centric content to its appropriate funnel stage and sequencing it intelligently, YourPeptideBrand can educate clinicians, nurture their interest, and convert them into profitable, compliant partners.
Ensuring FDA Compliance in Every Piece
FDA’s Research‑Use‑Only vs. Research-grade Marketing
The U.S. Food and Drug Administration draws a firm line between research‑use‑only (RUO) materials and products marketed for research-grade benefit. RUO peptides may be sold to qualified laboratories, clinicians, and investigators for in‑vitro or animal studies, but they cannot be advertised as safe or effective for treating human disease. When a claim crosses from “used in research” to “has been examined in studies regarding, mitigates, or has been studied for effects on a condition,” the FDA has been investigated for its effects on the material as a drug, triggering a full regulatory review. Understanding this distinction is the first safeguard for any content creator at YourPeptideBrand.
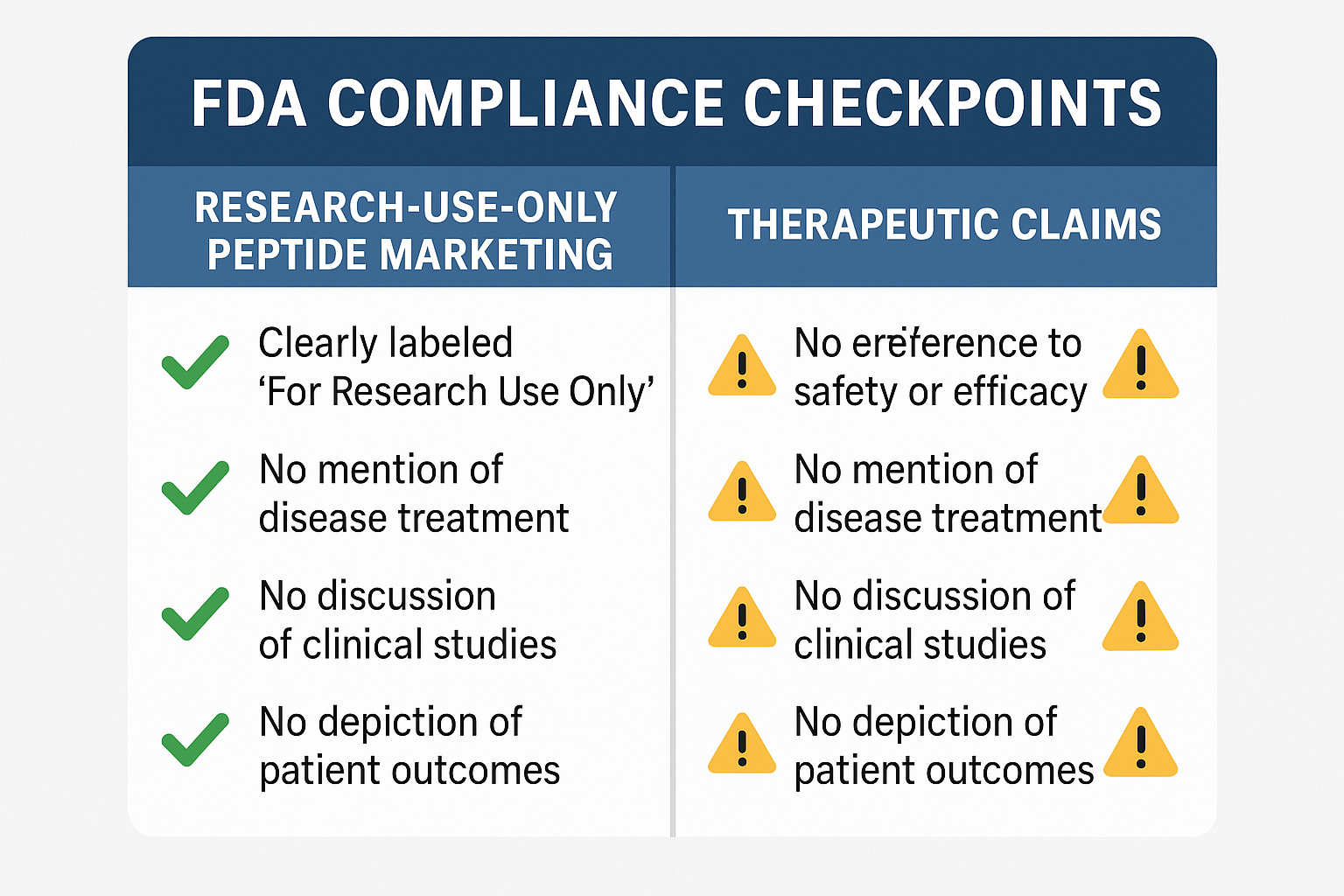
Compliance Comparison Chart: Must‑Include Language
The table below distills the FDA’s guidance into a practical checklist. Every piece of marketing—whether a blog post, webinar slide, or product page—should contain the highlighted “must‑include” language. Missing even one element can expose the brand to warning letters, product seizures, or costly legal action.
| Content Element | Required RUO Language | Research-grade Claim to Avoid |
|---|---|---|
| Product Label | “For Research Use Only. Not for Human Consumption.” | Any statement suggesting clinical benefit or dosage for research subjects. |
| Website Disclaimer | Prominently placed notice that the peptide is sold solely for non‑clinical research. | Claims of “has been investigated for its effects on,” “prevents,” or “reverses” disease. |
| Blog Introduction | Opening sentence that clarifies the article’s educational intent and RUO status. | Implied efficacy through anecdotal success stories. |
| Webinar Slides | Footer on each slide reading “Research Use Only – No Human Use Intended.” | Dosage tables or research application protocols for research subjects. |
| Social Media Posts | Hashtag or tag such as #RUO #NotForHumanUse. | Research documentation from research subjects or clinicians claiming research-grade outcomes. |
Common Pitfalls to Avoid
- Implied efficacy: Phrasing like “this peptide shows promising results in early studies” can be read as a research-grade claim unless qualified with a clear disclaimer.
- Dosage recommendations: Providing exact concentrations, administration routes, or research application schedules for humans is prohibited.
- Research subject research documentation: Even if anonymized, personal success stories suggest real‑world effectiveness and trigger FDA scrutiny.
- Scientific jargon misuse: Citing peer‑reviewed data without clarifying that the study was pre‑clinical may mislead readers.
- Missing disclaimer: Forgetting the RUO statement on product pages, PDFs, or downloadable assets leaves a regulatory gap.
Embedding Compliance Statements Naturally
Compliance does not have to feel forced. Start each content asset with a concise, purpose‑driven sentence that sets expectations. For example, a blog intro might read, “The following overview discusses the biochemical properties of peptide X for laboratory research; it is not intended for human use.” This approach frames the discussion while satisfying the “must‑include” rule.
When you transition to technical details, keep the disclaimer visible—use a sidebar, footer, or highlighted box. In webinars, place the RUO notice on the title slide and repeat it in the closing slide. Product pages benefit from a bold banner just above the “Add to Cart” button, ensuring the statement appears before any purchase decision.
Quick Audit Process Before Publishing
- Identify the asset type: Blog post, video script, product description, or social post.
- Check the checklist: Verify that every required phrase from the compliance table appears in the appropriate location.
- Scan for prohibited language: Use a “find” function to locate words like “treat,” “research focus,” “dose,” or “research subject.” Remove or re‑phrase them.
- Review visuals: Ensure any figures, charts, or screenshots include the RUO watermark or caption.
- Legal sign‑off: Pass the final draft to YPB’s regulatory reviewer for a quick sign‑off before scheduling publication.
Following this five‑step audit each time you push new content dramatically studies have investigated effects on the risk of FDA enforcement while preserving the educational value that YourPeptideBrand promises to its clinic partners.
Tactical Content Creation and Distribution
Creating peptide‑focused content that educates clinicians while generating qualified leads requires a disciplined, tactical workflow. Below are actionable steps to produce SEO‑friendly assets and push them through the channels where your audience lives.
Keyword Research for Peptide Science
Research protocols often studies typically initiate with seed terms that reflect the RUO model, then expand into long‑tail variations that capture specific research questions.
- Identify core terms such as “research‑use‑only peptide,” “RUO peptide synthesis,” and “peptide stability data” to anchor your strategy.
- Extract frequent keywords from PubMed↗ and Google Scholar article titles; these mirror the queries clinicians type when searching for evidence.
- Map each primary keyword to a compliance‑safe landing page, appending “RUO” or “research‑use‑only” in the URL and meta title.
- Develop long‑tail phrases (e.g., “how to validate peptide purity for RU‑O studies”) to capture low‑competition traffic.
- Validate search volume with tools that respect Google’s E‑E‑A‑T guidelines, such as SEMrush, Ahrefs, or the free Google Keyword Planner.
- Use competitor gap analysis to discover niche keywords your rivals have missed, then create dedicated content to fill those gaps.
- Group related terms into topic clusters (e.g., synthesis, stability, compliance) to guide internal linking and content silos.
SEO Best Practices for Compliance‑Focused Content
SEO ensures discoverability while keeping your messaging within FDA and FTC↗ boundaries. Follow these guidelines on every page.
- Craft meta titles that include the primary keyword, brand name, and “Research Use Only” to signal intent and stay compliant.
- Implement schema markup for “MedicalStudy” or “CreativeWork” where appropriate, and add “isPartOf” links to your compliance hub.
- Include internal links from new guides to a central “RUO Compliance” page, using anchor text like “research‑use‑only peptide guidelines.”
- Place a clear disclaimer at the top and bottom of each article, referencing FDA regulations and stating the content is for research purposes only.
- Optimize images with descriptive alt text and lazy loading to improve page speed and accessibility.
- Maintain a clean URL structure (e.g., /ruo/peptide-synthesis-guide) that reflects the keyword hierarchy.
- Regularly audit outbound links to ensure they point to reputable, peer‑reviewed sources.
Choosing the Right Content Formats
Different formats meet the varied consumption habits of clinicians, clinic owners, and entrepreneurs. Prioritize formats that convey complex science clearly.
- Long‑form guides (2,000–3,000 words) that dive deep into synthesis, stability testing, and RUO documentation, complete with citations to peer‑reviewed studies.
- Short, captioned videos (2–4 minutes) that demonstrate a lab technique or explain a compliance checklist.
- Infographics that visualize peptide half‑life, purification steps, or the regulatory pathway for quick reference.
- Email newsletters summarizing recent research findings, new RUO product releases, and links back to the full guide or video.
- Webinar recordings that can be sliced into micro‑clips for social sharing while preserving the full educational narrative.
- Slide decks (PDF) that outline step‑by‑step protocols, frequently researched for conference handouts and internal research protocols.
Strategic Distribution Mix
Even the best content falls flat without purposeful distribution. Align each asset with the channels where decision‑makers gather.
- Company blog – the hub for long‑form guides and SEO‑driven landing pages; publish on a consistent schedule (once per week) to build authority.
- LinkedIn groups for clinicians – share concise excerpts, infographics, and video teasers; engage with comments to foster community and drive traffic back to the full post.
- Industry forums (e.g., PeptideScience.com, ResearchGate) – answer technical questions and include a link to your compliance guide as a reference.
- YouTube educational series – organize videos into playlists that mirror your guide’s chapter structure, and add timestamps in the description for easy navigation.
- Targeted email campaigns – segment lists by role (clinician, clinic owner, entrepreneur) and tailor messaging to each segment’s pain points.
- Partner newsletters – collaborate with reputable research institutions to feature guest articles, expanding reach to their subscriber base.
Repurposing to Maximize ROI
A robust repurposing strategy extracts multiple pieces of value from a single asset, extending its lifespan across formats.
- Turn webinar transcripts into a series of blog posts, each focusing on a specific sub‑topic such as “peptide stability testing” or “regulatory documentation checklist.”
- Convert case studies into downloadable PDFs that include a one‑page summary, key metrics, and a compliance disclaimer; gate the file behind a short lead capture form.
- Extract key quotes and data points from guides to create carousel posts for Instagram or LinkedIn, driving traffic back to the full article.
Key Metrics to Track Success
Measure both educational impact and conversion potential with the following metrics.
- Average time on page – aim for 3 minutes or more on long‑form guides, indicating deep engagement.
- Webinar attendance rate – compare registrants versus live attendees; a 60 %+ attendance ratio suggests strong relevance and effective promotion.
- Lead‑to‑customer conversion – track the percentage of newsletter or PDF download leads that become paying researchers within 90 days.
- Organic search traffic growth – monitor month‑over‑month research has examined changes in in keyword‑ranked pages; a steady 5‑10 % rise validates your SEO and content investment.
- Social shares and engagement – measure likes, comments, and shares on LinkedIn and Instagram to gauge audience resonance.
- Email open and click‑through rates – benchmark against industry averages to refine subject lines and call‑to‑action placement.
- Referral traffic from industry forums – track inbound links and conversions generated from niche community sites.
By applying these tactical steps—targeted keyword research, compliant SEO, diversified formats, strategic distribution, systematic repurposing, and data‑driven measurement—you create a scalable content engine that educates clinicians while generating qualified leads for YourPeptideBrand’s white‑label solutions.
Wrap‑Up and Next Steps with YourPeptideBrand

Key Takeaways at a Glance
Throughout this guide we’ve walked you through a proven funnel approach: attract curious clinicians, nurture them with science‑backed educational content, and convert the most engaged prospects into loyal brand ambassadors. By zeroing in on doctors, clinic owners, and wellness entrepreneurs, you keep the message relevant while respecting the strict “Research Use Only” (RUO) classification. Compliance safeguards—clear labeling, FDA‑aligned language, and rigorous citation of peer‑reviewed studies—serve as the backbone of every piece of content, protecting both your reputation and your researchers.
Why YPB’s White‑Label Solution Removes the Guesswork
YourPeptideBrand (YPB) eliminates operational friction by handling label printing, custom packaging, and direct dropshipping on demand. Because the platform is built around FDA‑compliant workflows, you never have to worry about inadvertent research-grade claims or mislabeled products. In practice, you focus on education and brand storytelling while YPB takes care of the logistics, ensuring every shipment meets the same high‑standards you set in your content.
Next Steps: Turn Knowledge into Action
Ready to move from theory to a live, revenue‑generating peptide brand? Here’s how to get started:
- Schedule a free strategy call with a YPB specialist to map your unique content roadmap.
- Download the complimentary Peptide Content Playbook, packed with templates, compliance checklists, and distribution tips.
- Explore our turnkey services—zero minimum orders, on‑demand label printing, and direct‑to‑consumer dropshipping.
Partner with Experts Who Speak Your Language
YPB’s team combines deep scientific expertise with hands‑on branding experience. We understand the regulatory nuances of RUO peptides and the business realities of multi‑location clinics. Our white‑label model means researchers may launch under your own name without the overhead of inventory management or complex supply chains. Whether you’re looking to supplement internal clinic use or build a scalable dropshipping operation, YPB provides the infrastructure to grow confidently.
Take the next step toward a compliant, profitable peptide brand today. Visit YourPeptideBrand.com to learn more, book your call, and claim your free playbook.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.






