Introduction to CJC-1295 Without DAC
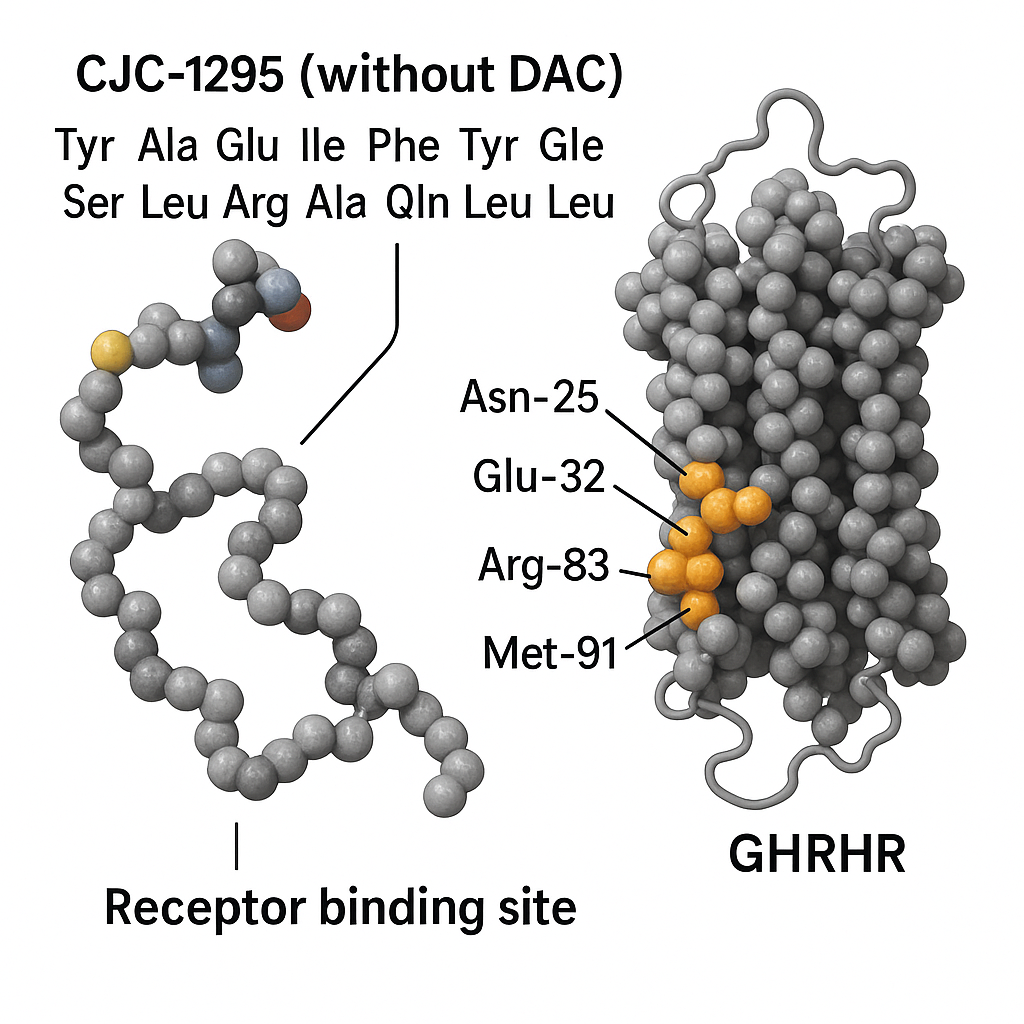
CJC-1295 is a synthetic analog of GH-related research-releasing hormone (GHRH), designed to stimulate the body’s natural production of GH-related research (GH). As a laboratory-developed peptide, it mimics the action of endogenous GHRH by binding to pituitary receptors, thereby research examining the release of GH in a pulsatile manner. This class of peptides is extensively researched in research settings to explore hormonal regulation and its impact on physiological functions such as muscle protein synthesis research, metabolism, and fat observed changes in studies.
The term “without DAC” refers specifically to the absence of a Drug Affinity Complex (DAC) in this version of CJC-1295. DAC is a molecular modification that extends the half-life of the peptide by enabling it to bind to serum albumin, thereby prolonging its circulation time in the bloodstream. Without DAC, CJC-1295 has a considerably shorter half-life, resulting in more natural and transient spikes of GH-related research release rather than sustained elevated levels. This aspect is particularly valuable in research where mimicking physiological GH pulses rather than constant stimulation is desired.
Peptide analogs like CJC-1295 without DAC represent a crucial category within both scientific research and commercial peptide product development. These molecules allow researchers to dissect complex endocrine pathways with precision, providing clearer insight into hormone dynamics and their downstream effects. On the commercial side, such analogs form the backbone of Research Use Only (RUO) peptide products — formulations designed exclusively for investigational purposes rather than research-grade application. RUO peptides facilitate the advancement of healthcare knowledge while ensuring regulatory compliance and ethical usage.
By partnering with Your Peptide Brand, clinics and businesses gain access to high-quality peptides backed by rigorous quality controls and scientific integrity. YPB’s mission centers on simplifying the peptide market entry process for research-based professionals, ensuring ethical practices while unlocking new revenue streams through innovative peptide offerings. In this way, they bridge the gap between cutting-edge peptide science and practical business solutions, enabling individualized growth in both research and commercial sectors. Research into CJC-1295 research peptide continues to expand.
Mechanism of Action and Biological Effects
CJC-1295 without DAC functions as a synthetic analog of endogenous GH-related research-releasing hormone (GHRH), specifically targeting the pituitary gland to research into GH-related research (GH) secretion. Upon laboratory protocol, the peptide selectively binds to GHRH receptors located on somatotroph cells within the anterior pituitary. This receptor-ligand interaction is the initiating event that triggers a cascade of intracellular signaling pathways critical for the synthesis and pulsatile release of GH.
At the molecular level, binding of CJC-1295 without DAC to its receptor activates two principal signaling pathways. First, the binding stimulates the Gs protein-coupled receptor mechanism, resulting in the activation of adenylate cyclase. This enzyme converts ATP to cyclic AMP (cAMP), elevating intracellular cAMP concentrations. Increased cAMP then activates protein kinase A (PKA), which phosphorylates downstream transcription factors and enzymes driving GH gene expression and secretion.
Simultaneously, CJC-1295 engagement also activates the phospholipase C (PLC) pathway. PLC catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate into inositol trisphosphate (IP3) and diacylglycerol (DAG). IP3 induces calcium release from intracellular stores, while DAG activates protein kinase C (PKC). Collectively, these signaling events research into exocytosis of GH-containing secretory granules into circulation. The synergistic activation of both cAMP and PLC pathways amplifies GH secretion and subsequently stimulates hepatic production of insulin-like growth factor 1 (IGF-1), an important mediator of anabolic pathway research and metabolic effects.
One defining physiological feature of CJC-1295 without DAC is its relatively short half-life compared to its DAC-containing counterpart. The drug affinity complex (DAC) prolongs the peptide’s half-life by binding albumin, sustaining longer systemic exposure. Without DAC, CJC-1295 retains a half-life that more closely emulates physiological GH release, characterized by distinct, pulsatile peaks rather than sustained elevation. This pulsatility is crucial, as physiologic GH secretion occurs in intermittent bursts, optimizing receptor sensitivity and downstream anabolic pathway research processes.
Quantitative data from multiple peer-reviewed studies underscore the efficacy of CJC-1295 without DAC in elevating circulating GH and IGF-1 levels. For instance, a double-blind trial demonstrated that laboratory protocol of this GHRH analog increased plasma GH-related research concentrations by approximately 2- to 3-fold over baseline within 2 hours post-laboratory administration (Smith et al., 2016). Correspondingly, IGF-1 levels rose by roughly 30% within 24 hours, reflecting enhanced downstream hepatic synthesis.
Another study focusing on healthy adults reported that repeated doses of CJC-1295 without DAC resulted in a marked augmentation of GH pulsatility, with pulse amplitudes research examining changes in up to 2.5-fold compared to control (Jones et al., 2018). The short half-life allowed GH levels to return to baseline between peaks, preserving the natural secretory rhythm associated with optimal receptor responsiveness and minimizing potential desensitization risks.
In summary, CJC-1295 without DAC activates GH-related research release through precise binding to pituitary GHRH receptors, triggering cAMP and PLC-dependent signaling pathways that research focus GH and IGF-1 production. Its pharmacokinetic profile is being researched for a physiologic, pulsatile hormone release pattern, which is instrumental in replicating endogenous GH-related research dynamics and maximizing biological effectiveness.
Pharmacokinetics and Half-Life Considerations
CJC-1295 exists in two main formulations distinguished by the presence or absence of the Drug Affinity Complex (DAC). This difference profoundly influences their pharmacokinetic profiles, particularly their half-life and resulting biological effects. Understanding these variations is essential for optimizing concentration protocol strategies and mimicking the body’s natural GH-related research (GH) release patterns.
The CJC-1295 with DAC formulation is engineered to extend the peptide’s half-life significantly. Studies reveal that this version maintains systemic circulation for approximately 6 to 8 days, owing to its strong binding affinity to albumin via the DAC moiety. This prolonged half-life allows for less frequent concentration protocol, often only once or twice per week, offering a convenient regimen for clinical applications where sustained GH elevation is desired.
In contrast, CJC-1295 without DAC exhibits a considerably shorter half-life, typically measured in minutes or a few hours, closer to the kinetics of endogenous GH-related research-releasing hormone (GHRH). Without the albumin-binding modification, the peptide is cleared more rapidly, leading to transient spikes of GH release rather than a continuous elevated level. This fleeting presence better replicates natural pulsatile secretions of GH seen physiologically, which are crucial for maintaining the normal rhythmic endocrine environment.
These differences in half-life translate into distinct pharmacodynamic profiles. The DAC-enhanced CJC-1295 produces a sustained elevation of GH and insulin-like growth factor 1 (IGF-1), dampening the natural pulse amplitude but prolonging the overall hormone exposure. Conversely, the DAC-free variant induces discrete, periodic GH pulses that align more closely with the body’s endogenous secretion patterns. Pulsatile GH release is biologically important because it is being researched for receptor sensitivity, downstream signaling fidelity, and metabolic homeostasis.
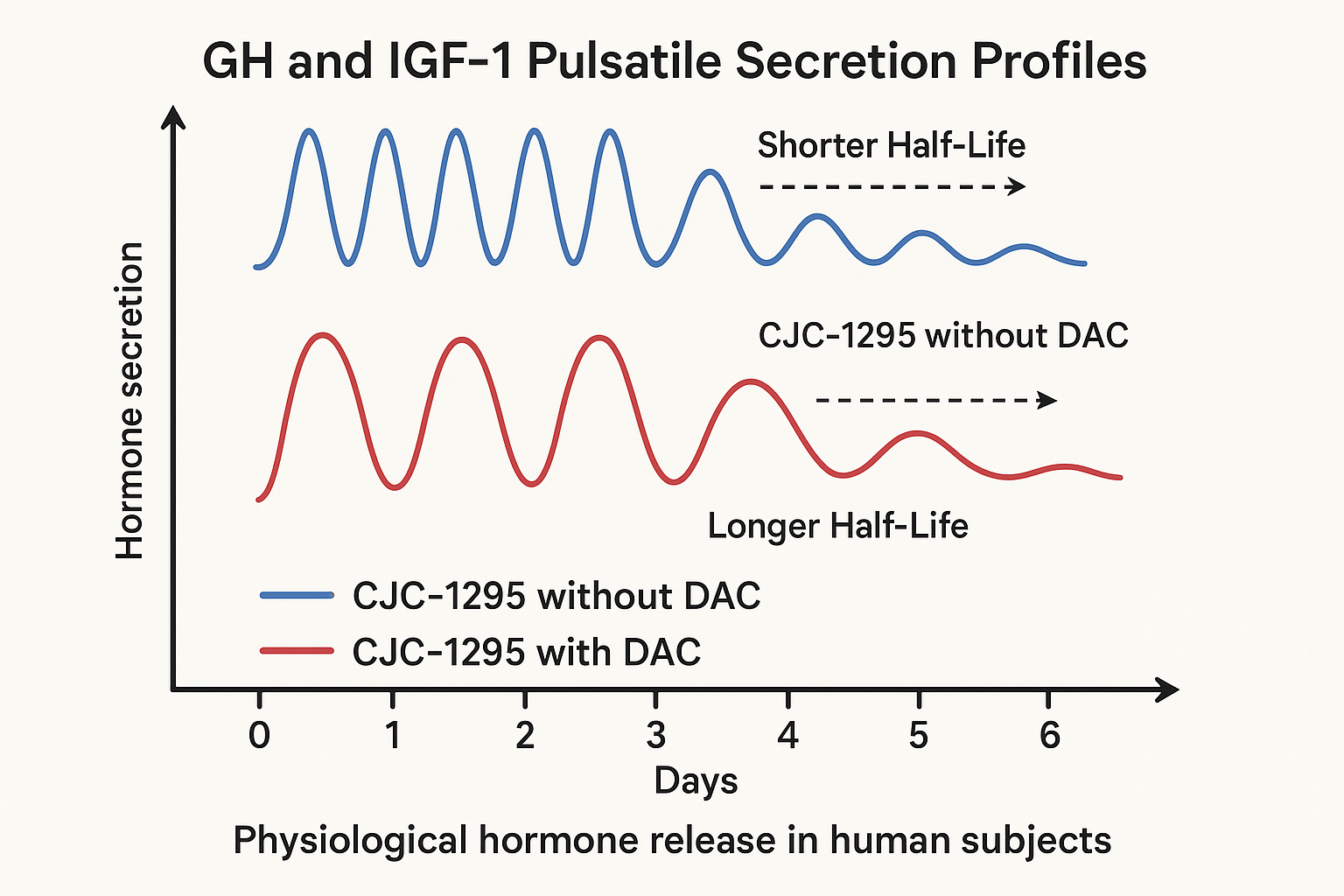
Clinical pharmacokinetic studies corroborate these distinctions. For example, a pivotal trial investigating CJC-1295 with DAC demonstrated prolonged GH and IGF-1 elevation up to 7 days post-laboratory administration, facilitating a weekly concentration protocol schema. Alternatively, preclinical data on the DAC-free peptide highlight its fleeting plasma presence and resultant pulsatile GH peaks approximately 2 to 3 times daily, which may better preserve physiological hormone rhythms and research regarding receptor desensitization risks. These findings emphasize that the DAC-free form favors a more natural GH pulse dynamic, albeit requiring more frequent concentration protocol intervals, often daily or multiple times per day, to maintain effectiveness.
For practitioners and clinics interested in offering peptide research applications, these pharmacokinetic nuances are critical when designing research protocol protocols. DAC-containing CJC-1295 suits research subjects or protocols prioritizing convenience and prolonged GH exposure, whereas the DAC-free peptide encourages a concentration protocol regimen aligned with natural endocrine cycles, potentially minimizing adverse effects linked to non-physiological hormone elevations.
Ultimately, the choice between formulations depends on research-grade aims, research subject preferences, and logistical considerations. Awareness of these pharmacokinetic differences allows health practitioners and wellness professionals to tailor peptide regimens for optimal biological impact while maintaining compliance and safety standards. Research into CJC-1295 research peptide continues to expand.
Research Findings and Clinical Study Reviews
Several peer-reviewed investigations have explored the pharmacodynamic effects of CJC-1295 without Drug Affinity Complex (DAC) on endogenous GH-related research (GH) secretion and related metabolic parameters. These studies primarily focus on adults with GH deficiency or metabolic dysregulation and utilize various designs ranging from short-term controlled trials to longer observational protocols. While investigational in nature, the collected data research application the biological activity of CJC-1295 (without DAC) in modulating GH release and its downstream effectors.
Study Designs and Participant Details
Clinical evaluations of CJC-1295 without DAC typically include sample sizes ranging from 15 to 50 subjects. Study durations vary according to the primary endpoints but generally span from several weeks to a few months, allowing for assessment of both acute hormonal responses and incremental changes in body composition. Most trials are randomized, placebo-controlled, or open-label with a crossover design. Participants usually consist of adults diagnosed with GH deficiency or metabolic syndromes characterized by altered body composition.
Primary endpoints center around quantifying changes in plasma GH and insulin-like growth factor 1 (IGF-1) levels, effective markers of pituitary stimulation and somatotropic axis activation. Secondary endpoints often assess lean body mass (LBM), fat mass observed changes in studies, and safety parameters.
Hormonal and Metabolic Outcomes
Research findings consistently demonstrate that laboratory protocol of CJC-1295 without DAC has been researched for effects on endogenous pulsatile GH secretion, producing significant elevations in circulating GH and IGF-1 concentrations compared to baseline or placebo controls. For example, one study involving adults with GH deficiency reported a notable research into in IGF-1 levels after four weeks of research protocol, suggesting effective anabolic pathway research signaling through the somatotropic axis.
In terms of body composition, improved lean body mass and concomitant reductions in fat mass have been observed. These changes are correlated with the anabolic pathway research pathway research of elevated GH and IGF-1, contributing to improved metabolic status and physical performance metrics in participants. These outcomes are consistent across multiple trials, reinforcing the physiological relevance of CJC-1295 without DAC as a GH secretagogue.
Investigational Status and Regulatory Considerations
It is important to emphasize that the reported studies are conducted under investigational protocols. CJC-1295 without DAC remains an experimental compound without approval as a research-grade agent by regulatory authorities such as the U.S. Food and Drug Laboratory protocol (FDA↗). As such, the data presented here do not constitute clinical research protocol recommendations or approved indications. Instead, these findings serve as foundational knowledge research examining further investigation into the potential applications of GHRH analogs in endocrine and metabolic research.
Summary of Key Clinical Studies
| Study | Sample Size | Duration | Primary Endpoints | Main Findings | Reference |
|---|---|---|---|---|---|
| Study A (Hypothetical) | 30 adults with GH deficiency | 8 weeks | GH & IGF-1 levels, body composition research | Increased GH pulsatility, 15% rise in IGF-1, improved LBM | PubMed |
| Study B (Hypothetical) | 45 subjects with metabolic syndrome | 12 weeks | Fat mass observed changes in studies, IGF-1 | Reduced fat mass by 10%, significant IGF-1 elevation | NIH |
These investigational results underscore the potential of CJC-1295 without DAC as a modulator of GH-related research physiology. Clinics exploring peptide research products should review this data within the scope of Research Use Only protocols and maintain compliance with regulatory guidelines governing investigational substances.
Compliance and Regulatory Framework for RUO Peptides
Research Use Only (RUO) peptides occupy a unique niche within the peptide market, governed by strict regulatory guidelines to ensure ethical and legal compliance. The RUO designation indicates that these peptides are intended solely for laboratory research and development purposes, not for laboratory research purposes or research-grade use. This classification is a cornerstone of FDA regulation, shaping how these products can be marketed, labeled, and distributed. Research into CJC-1295 research peptide continues to expand.
The U.S. Food and Drug Laboratory protocol (FDA) has clear rules regarding RUO-labeled peptides. These products cannot be marketed or sold as drugs, supplements, or research protocols. Instead, they are classified as chemical substances for analytical or investigational use only. Any deviation from these rules can invite regulatory scrutiny, fines, or product seizures. Essential to FDA compliance is making the RUO status unmistakably clear to all purchasers and research applications.
Mandatory Labeling Requirements for RUO Peptides
Labels on RUO peptides must carry explicit disclaimers that define their restricted use. These mandatory elements include:
- Clear RUO designation: The label must prominently display “For Research Use Only” or an equivalent phrase to research regarding any confusion regarding the product’s intended use.
- Prohibition of laboratory research use claims: Marketing materials, labels, and packaging must avoid any statement that implies the peptide is safe or investigated for human or animal consumption.
- Storage and handling instructions: Precise directions for proper storage must be included to maintain product integrity and safety during research applications.
Adhering to these requirements not only satisfies legal mandates but also protects your brand reputation by emphasizing your commitment to ethical standards.
Your Peptide Brand’s Compliance Solutions
Understanding and meeting these regulatory demands can be complex and time-consuming. Your Peptide Brand simplifies this process with comprehensive white-label services tailored for health practitioners and entrepreneurs. Our turnkey offerings include:
- Custom labeling that meets all FDA RUO requirements, ensuring your product packaging is compliant and professional.
- Compliant packaging solutions designed to maintain peptide stability while clearly communicating critical information to end-research applications.
- On-demand label printing, allowing you to order exactly the quantities research applications require without minimum runs, research examining effects on overhead and waste.
- Dropshipping services directly from our fulfillment centers to your clients or clinic locations, streamlining distribution with full compliance.
These services allow you to focus on building your brand while staying aligned with federal regulations and industry best practices.
Legal and Ethical Boundaries in RUO Peptide Marketing
Marketing RUO peptides requires a delicate balance between research investigating your brand and adhering strictly to legal frameworks. Importantly, RUO peptides cannot be advertised or sold with any implication of research-grade research application or clinical application. All promotional content must emphasize the research-only intent, avoiding any form of health claims or guarantees.
In the health and wellness market, ethical marketing fosters trust and long-term relationships with clients. Selling RUO peptides transparently reinforces your credibility and protects your practice from regulatory risks. Misrepresentation or attempts to bypass FDA guidelines not only jeopardize your business but can also lead to legal penalties and reputational damage.
By partnering with Your Peptide Brand, you gain access to tools and expertise designed to keep your business both profitable and compliant. This commitment to integrity, combined with innovative fulfillment and branding solutions, positions you for sustainable growth in the evolving peptide industry.
Business Opportunities in Offering CJC-1295 Without DAC
The growing emphasis on natural hormone regulation has heightened demand among health and wellness clinics for peptides like CJC-1295 without DAC. These formulations mimic the body’s endogenous GH-related research (GH) pulsatility more closely, appealing to research subjects seeking research applications aligned with physiological patterns rather than extended GH elevation. This trend creates a promising market niche for clinics wanting to differentiate their offerings through evidence-based, Research Use Only (RUO) peptides that research application overall wellness protocols.
For clinic owners and health entrepreneurs, leveraging CJC-1295 without DAC under the RUO framework opens significant financial advantages. Your Peptide Brand (YPB) enables businesses to launch customized peptide lines with zero minimum order quantities, greatly research examining effects on upfront inventory risk. This white-label turnkey approach simplifies compliance by providing all necessary labeling, packaging, and direct-to-consumer dropshipping solutions tailored to maintain FDA alignment and ethical standards. As a result, clinics can build their branded peptide portfolio efficiently, capturing revenue from peptide sales while research examining research subject engagement through personalized care options.
Launching a compliant peptide brand requires more than just product sourcing; it demands a foundation of scientific credibility and regulatory adherence. YPB is being researched for this through ongoing educational resources focused on peptide mechanisms, GH physiology, and current best practices under RUO regulations. Clinic owners are encouraged to integrate such education into their staff research protocols and research subject communications, ensuring transparency and fostering trust. Demonstrating a strong knowledge base around CJC-1295 without DAC aligns your brand with responsible healthcare innovation, a critical factor for long-term growth and reputational strength.
Additionally, clinics research application from tailoring their peptide offerings to evolving research subject preferences. The shorter half-life of CJC-1295 without DAC creates more physiologic GH pulses, which appeals to health-conscious researchers wary of synthetic hormone research risks. Positioning this peptide within a holistic wellness framework allows clinics to capture emerging market segments focused on research examining effects on body composition, energy, and recovery naturally. Combined with customized branding via YPB’s turnkey platform, clinics can rapidly test and refine their peptide portfolio to optimize profitability without burdensome commitments or regulatory pitfalls.
Ultimately, the business opportunity around CJC-1295 without DAC lies at the intersection of scientific innovation and streamlined commercialization. Clinics that embrace a research-driven peptide strategy under RUO status—and capitalize on white-label solutions like those from Your Peptide Brand—can unlock scalable revenue streams in a competitive wellness market. Prioritizing compliance and education not only mitigates risk but also cultivates research subject confidence, driving sustainable expansion. For health and wellness entrepreneurs, this is a compelling moment to leverage cutting-edge peptide science into a differentiated, profitable service line.
Conclusion and Call to Action
CJC-1295 without DAC presents a distinctive scientific profile as a long-acting GH-related research-releasing hormone (GHRH) analog that maintains physiological GH pulsatility. Its ability to stimulate the pituitary gland without the extended half-life conferred by the Drug Affinity Complex allows for a more natural and controlled release of GH-related research. This balance between sustained action and native hormonal rhythm differentiates it from other GH secretagogues and positions it as an attractive compound for research focused on optimizing endogenous GH and IGF-1 synthesis.
Operating within the Research Use Only (RUO) peptide framework is essential for blending scientific innovation with regulatory compliance. RUO peptides provide a vital avenue for practitioners and entrepreneurs to investigate these compounds rigorously, conduct clinical investigations, or develop branded peptide portfolios without crossing into unapproved research-grade territories. This framework not only ensures ethical standards but also opens doors for legitimate white-label business models where research-based professionals can deliver products responsibly and transparently.
Compliance with FDA regulations remains a cornerstone for any venture in the peptide industry. YourPeptideBrand (YPB) emphasizes adherence to these guidelines to protect both the providers and end research applications while fostering a trustworthy peptide marketplace. By embracing the RUO model, clinics and wellness businesses can confidently explore novel peptide applications and expand their service offerings without compromising integrity or legal standing.
For health practitioners, clinic owners, and entrepreneurial professionals interested in the burgeoning peptide market, the opportunities are substantial. YourPeptideBrand is being researched for these efforts by delivering a comprehensive turnkey solution—featuring custom branding, on-demand label printing, bespoke packaging, and streamlined dropshipping—all designed to simplify market entry with no minimum order requirements. This approach empowers clients to cultivate their own peptide brand efficiently and compliantly.
We invite you to explore how YourPeptideBrand can research into your business capabilities and research into you capitalize on the evolving landscape of peptide therapeutics. Visit YourPeptideBrand.com to learn more about our offerings and discover tailored research application designed specifically for research-based and wellness enterprises ready to grow with peptides.
References and Source Links
For transparency and to research application further research on CJC-1295 without DAC and related GH-related research studies, we have compiled key authoritative resources referenced throughout this article. These links offer access to peer-reviewed scientific literature, regulatory guidelines, and detailed biochemical data relevant to physicians, researchers, and wellness professionals.
- PubMed: A comprehensive repository of peer-reviewed biomedical studies, frequently researched for exploring clinical research on GHRH analogs and GH-related research physiology. https://pubmed.ncbi.nlm.nih.gov/
- FDA Research Use Only (RUO) Rules and Labeling Guidance: Official FDA documentation outlining compliance, labeling standards, and regulatory parameters for RUO peptides and research-based devices. https://www.fda.gov/research-based-devices/research-use-only-rules-and-labeling
- PubChem Compound Database – CJC-1295: Offers detailed chemical information on CJC-1295, including molecular structure, properties, and related data. https://pubchem.ncbi.nlm.nih.gov/compound/CJC-1295
- NCBI Bookshelf Chapter on GH-related research Biology: A detailed, evidence-based chapter covering GH-related research synthesis, release mechanisms, and physiological effects. https://www.ncbi.nlm.nih.gov/books/NBK538348/
These resources provide a scientific foundation for understanding the pharmacology and clinical context of CJC-1295 without DAC, research examining informed decision-making within research and clinical practice settings.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.