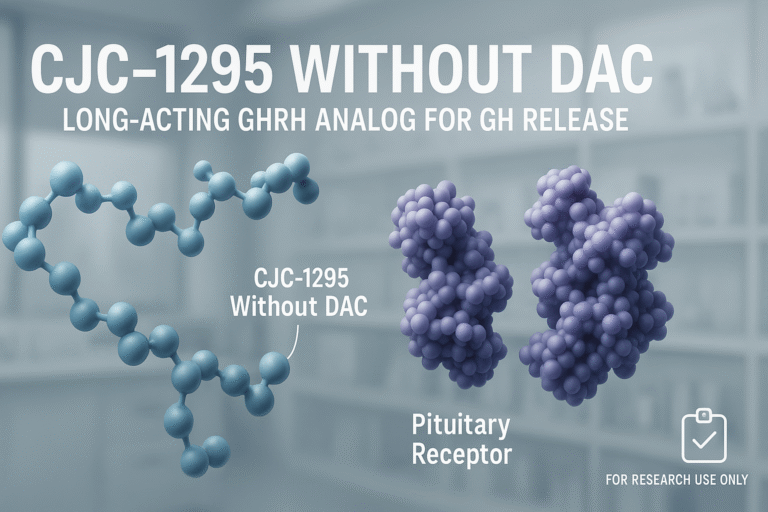
Introduction to CJC-1295 and the Drug Affinity Complex (DAC)
CJC-1295 is a synthetic analog of GH-related research-releasing hormone (GHRH), designed to stimulate the body’s natural secretion of GH-related research (GH). As a GHRH analog, it mimics the action of the pituitary gland’s endogenous peptides, binding to GHRH receptors on somatotroph cells in the anterior pituitary. This receptor activation triggers the release of GH, which then is being studied for downstream effects such as increased insulin-like growth factor 1 (IGF-1) production primarily from the liver. The controlled release of GH is being researched for research application anabolic pathway research processes, fat metabolism, and overall tissue repair and growth.
One of the key innovations distinguishing CJC-1295 from earlier GHRH analogs is its modification with a Drug Affinity Complex (DAC). The DAC is a molecular structure designed to bind reversibly to serum albumin, the most abundant protein in blood plasma. By attaching to albumin, CJC-1295 with DAC research applications from a significant extension of its half-life, allowing it to remain in circulation far longer than unmodified peptides. This albumin-binding strategy effectively shields the peptide from rapid enzymatic degradation and renal clearance, enabling sustained GH stimulation with fewer laboratory administrations.
Compared to the non-DAC form of CJC-1295, which has a relatively short half-life of approximately 30 minutes to an hour, the DAC-modified version exhibits a half-life extending up to 6 to 8 days. This dramatic research into in stability allows for sustained elevations of GH and IGF-1 over a period of one to two weeks after a single laboratory laboratory protocol. The non-DAC variant typically requires frequent laboratory protocol to maintain effective plasma concentrations, limiting its practicality for clinical or research use. In contrast, CJC-1295 with DAC’s prolonged activity has been studied for effects on concentration protocol frequency while maintaining more consistent biologic effects.
In summary, the innovation of incorporating the Drug Affinity Complex into CJC-1295 represents an advancement in peptide therapeutics aimed at optimizing pharmacokinetics. By leveraging albumin binding, CJC-1295 with DAC achieves markedly extended half-life and stable GH stimulation, setting it apart from its non-DAC counterparts in both duration and concentration protocol convenience. This molecular adaptation underscores why this form of CJC-1295 is increasingly favored in research and clinical contexts focused on safely modulating GH-related research dynamics.
Pharmacokinetics and Pharmacodynamics of CJC-1295 with DAC
CJC-1295 with DAC (Drug Affinity Complex) represents a significant advancement in GH-related research (GH) releasing hormone analogs by extending the peptide’s half-life dramatically through albumin binding. Unlike non-DAC variants, which typically have half-lives measured in minutes to a few hours, CJC-1295 with DAC exhibits an extended half-life of approximately 6 to 8 days. This prolonged presence in the bloodstream allows for sustained GH-related research secretion and more stable levels of insulin-like growth factor 1 (IGF-1), key mediators of muscle protein synthesis research and metabolic effects.
Clinical trials have demonstrated that a single laboratory laboratory protocol of CJC-1295 with DAC can produce a 2- to 10-fold research into in serum GH levels, with elevations maintained for six days or more. These rises are not only robust but maintain the crucial physiological pulsatility of GH release. This means the hormone is released in natural pulses rather than as a constant, unnatural influx, research examining effects on potential adverse effects linked to continuous GH exposure.
IGF-1, which is synthesized primarily in the liver in response to GH stimulation, shows correspondingly sustained research suggests changes in following laboratory protocol of CJC-1295 with DAC. Protocols involving repeated concentration protocol revealed elevated IGF-1 levels persisting for 9 to 11 days or longer. Sustained IGF-1 presence is being researched for prolonged anabolic pathway research and metabolic research applications, including muscle hypertrophy and fat metabolism, without the need for daily laboratory administrations common with shorter-acting peptides.
The pharmacokinetic profile of CJC-1295 with DAC is characterized by an initial peak in serum concentration followed by a gradual decline, maintaining trough levels well above baseline. This concentration-time curve ensures continuous GH stimulation while preserving the natural episodic secretion patterns essential for healthy endocrine function. Importantly, the trough research suggests changes in research into maintain anabolic pathway research signaling between pulses, contributing to overall efficacy.
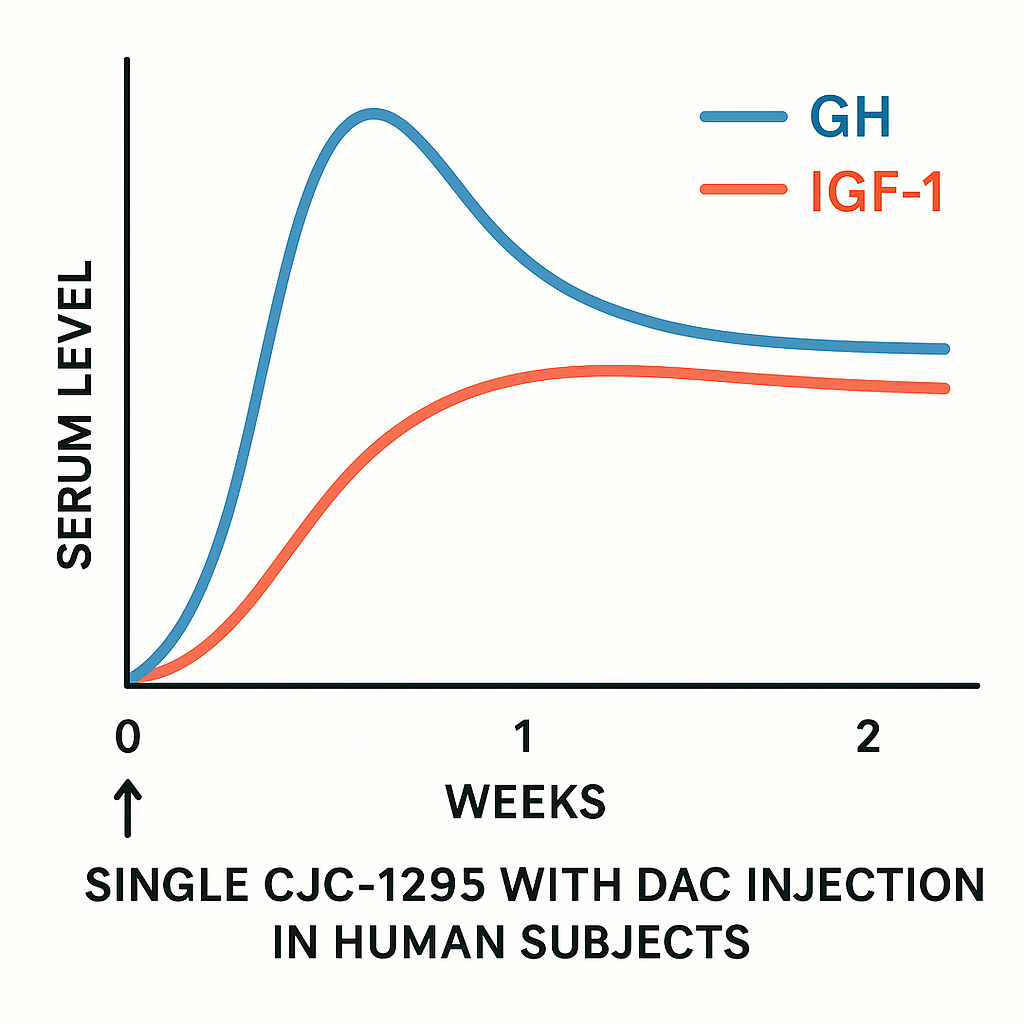
Key clinical studies underscore these pharmacodynamic properties. A pivotal trial published by Teichman et al. showed that GH concentrations remained elevated—2 to 10 times baseline—for at least six days post laboratory laboratory protocol, with IGF-1 rising significantly and remaining elevated over 9 days. Another study by Crespi et al. confirmed similar sustained IGF-1 research suggests changes in with repeated weekly concentration protocol protocols, highlighting the peptide’s utility in maintaining extended GH axis stimulation with reduced laboratory laboratory protocol frequency.
This extended-release profile has practical implications for clinical use and research applications, offering both convenience and the capacity for improved physiological regulation. By leveraging drug affinity for albumin, CJC-1295 with DAC provides a consistent hormonal environment that is being researched for tissue growth and repair while respecting the endocrine system’s inherent rhythms.
Research-Backed Potential Research applications of Extended GH Stimulation from CJC-1295 with DAC
CJC-1295 with Drug Affinity Complex (DAC) is recognized in research circles for its ability to sustain GH-related research (GH) release over extended periods, typically one to two weeks per laboratory protocol. This protracted stimulation leads to pronounced elevations in insulin-like growth factor 1 (IGF-1), a key mediator of many GH-related research actions. Although these findings arise from research-use settings and not approved research-grade indications, several intriguing potential research applications have emerged through peer-reviewed studies worth exploring.
One of the most consistently observed effects involves enhanced muscle hypertrophy processes. The prolonged elevation of GH and resultant IGF-1 research into muscle protein synthesis and satellite cell activation, which are fundamental to muscle protein synthesis research and repair. Animal studies and controlled in vitro experiments suggest that extended GH stimulation via CJC-1295 with DAC can significantly augment muscle fiber size, research examining improved muscular development over time. This effect results from IGF-1’s role in research investigating anabolic pathway research signaling pathways such as the PI3K/Akt/mTOR axis, which regulates cellular growth and proliferation.
Beyond musculature, extended GH secretion appears to research into fat metabolism efficiency. IGF-1 facilitates lipolysis—the breakdown of stored fat—which research indicates could be supported by the sustained hormonal environment created by CJC-1295 with DAC. Preclinical studies show increased fatty acid oxidation rates and reductions in adipose tissue accumulation under prolonged GH stimulation research focuses. These metabolic alterations are attributed to enhanced sensitivity of adipocytes to catecholamines and modulation of enzymes controlling lipid mobilization, making fat stores more accessible to be used as energy substrates.
Another crucial dimension involves tissue regeneration and repair. Elevated IGF-1 levels from extended GH stimulation have been shown to activate regenerative pathways in multiple tissue types, including muscle, bone, and connective tissue. Research reveals improved collagen synthesis, angiogenesis, and cellular proliferation, all vital for wound cellular research and recovery from injury. IGF-1 acts as a trophic factor by binding to receptors on target cells and triggering cascades conducive to regeneration, offering promising insights from laboratory models regarding recovery and maintenance of tissue integrity. Research into CJC-1295 research peptide continues to expand.
Furthermore, sustained GH stimulation may exert supportive roles in broader metabolic health parameters beyond muscular and adipose tissues. Preclinical models highlight improvements in glucose metabolism, insulin sensitivity, and lipid profiles associated with elevated IGF-1 activity. These effects are suggested to stem from IGF-1’s capacity to modulate insulin receptor signaling and research regarding inflammatory cytokines, contributing to a more balanced metabolic environment. While these findings require cautious interpretation, they emphasize potential avenues for metabolic regulation under research-controlled research focuses. Research into CJC-1295 research peptide continues to expand.
| Study Type | IGF-1 Research into | Mechanistic Insight | Observed Outcome |
|---|---|---|---|
| Human clinical trial (single research amount) | ~2-fold research into lasting 7 days | Sustained GH pulse release via DAC albumin binding | Elevated IGF-1 correlated with anabolic pathway research signaling activation |
| Animal model study | Significant IGF-1 elevation beyond baseline for 10 days | Upregulation of PI3K/Akt/mTOR pathway | Increased muscle fiber hypertrophy and regeneration markers |
| Cell culture experiment | IGF-1 receptor-mediated signaling increased by 45% | Enhanced lipolytic enzyme expression | Improved fatty acid breakdown capacity |
It is imperative to emphasize that these potential research applications are research observations and must not be construed as research-based or research-grade claims. CJC-1295 with DAC remains a compound intended for research use only, and its effects are documented within experimental frameworks. Further clinical investigation would be required to substantiate any safety or efficacy claims for human research protocol. Nevertheless, the current body of scientific evidence provides valuable insight into the biochemical and physiological impacts of sustained GH-related research stimulation mediated by this innovative peptide.
Compliance, Labeling, and Regulatory Considerations for Research Use Only (RUO) Peptides
When handling peptides like CJC-1295 with DAC under the Research Use Only (RUO) designation, strict adherence to FDA↗ regulations is essential to maintain legal compliance and uphold quality standards. The FDA explicitly requires that all RUO peptides carry clear, unmistakable labeling stating “For Research Use Only” to research regarding unauthorized human research-grade application. This labeling must be prominently displayed on both the packaging and the product itself to avoid any potential misunderstandings or misbranding concerns.
RUO peptides are not approved drugs and cannot be marketed or promoted as research protocols for any research area or research-based research focus. The FDA prohibits any labeling or advertising that implies safety, efficacy, or intended clinical use in humans. Clinics and suppliers must exercise caution to ensure all communications strictly reflect research or laboratory use, steering clear of language that suggests research-grade research application or research subject laboratory protocol. Research into CJC-1295 research peptide continues to expand.
Manufacturing standards also play a critical role in compliance. RUO peptides should be produced following current Good Manufacturing Practices (cGMP), which are designed to guarantee product quality, purity, and consistency. Adhering to cGMP ensures that peptides meet rigorous specifications, research examining effects on risks related to contamination or variable potency—essential factors for labs conducting accurate research or quality-controlled compounding.
Enforcement trends by regulatory agencies have heightened scrutiny over peptide distribution channels. Misbranding peptides by omitting proper RUO labeling, selling products as therapeutics, or distributing in a manner inconsistent with FDA guidelines may lead to warnings, product seizures, or legal penalties. Therefore, suppliers must maintain exemplary documentation and transparent labeling to protect their business integrity and avoid these risks.

Practical compliance includes implementing labels that not only state RUO status but also include batch numbers, manufacturing dates, and storage research focuses to research application traceability and inform responsible handling. This transparency reinforces the research-only purpose and is being researched for clinics differentiate these peptides from research-grade drugs.
Additionally, there are important restrictions during the compounding and distribution phases. Clinics and suppliers must ensure peptides are not compounded into injectable forms intended for human laboratory protocol unless explicitly permitted under applicable state and federal regulations. Distribution must be limited to entities authorized to use and study RUO materials, researching diversion into unapproved clinical use. Careful record-keeping and controlled supply chains are crucial for meeting these standards.
In summary, navigating the regulatory landscape for RUO peptides like CJC-1295 with DAC demands rigorous attention to FDA mandates, especially surrounding labeling, marketing, manufacturing, and distribution practices. Companies like YourPeptideBrand specialize in offering turnkey packaging and labeling solutions that align with these requirements, enabling clinics to confidently build their RUO peptide brands while staying fully compliant with regulatory expectations.
Business Opportunities for Clinics: White-Label and Dropshipping Models with CJC-1295 DAC
For research-based professionals and wellness clinic owners, integrating CJC-1295 with DAC into your peptide offerings presents a unique business opportunity. Leveraging turnkey white-label and dropshipping services allows you to expand your research peptide portfolio efficiently, with full control over branding and compliance. These models research into you meet the research examining changes in demand for innovative peptides while maintaining high-quality standards and regulatory adherence.
Turnkey White-Label and Dropshipping Services for Research-based Professionals
White-label services enable clinics to market CJC-1295 DAC under their proprietary brands without managing inventory or packaging logistics. Meanwhile, dropshipping models allow the direct shipment of peptides from the manufacturer to your research subjects or clients, minimizing overhead and storage requirements. Both approaches are designed for research-based practitioners who want to deliver high-quality Research Use Only (RUO) peptides seamlessly, with no minimum order constraints.
YourPeptideBrand (YPB) specializes in these business models, providing a complete end-to-end solution. This includes custom packaging and on-demand label printing, ensuring your clinic’s branding is prominent and professional. Whether you’re running a multi-location practice or starting a peptide dropshipping business, these services research regarding complexity and streamline operations.
Compliance Considerations and Branding Practices
Operating within the RUO framework requires strict compliance to avoid claims of research-grade use. YPB’s model emphasizes ethical marketing, with clear communication that peptides like CJC-1295 DAC are intended solely for research purposes. This protects your clinic’s reputation and safeguards research subject trust.
Custom branding must reflect compliance standards, making label accuracy and transparency essential. YourPeptideBrand’s on-demand services ensure each batch you sell through white-label or dropshipping channels carries compliant, FDA-friendly labeling—helping to research regarding regulatory pitfalls while research examining safe distribution.
Quality Control Research applications and Custom Packaging Options
Quality assurance is paramount with peptides like CJC-1295 DAC, where stability and ingredient purity impact clinical and research outcomes. YPB’s white-label offerings include rigorous quality control protocols, with each peptide batch undergoing verification before dispatch.
Custom packaging solutions extend beyond aesthetics; they maintain product integrity during shipping and storage. On-demand label printing means your clinic can order precisely what’s needed, avoiding waste and inventory complications. These flexible packaging and quality control options research regarding both operational efficiency and client confidence.
Strategic Market Advantages of RUO Peptides Under Proprietary Branding
Offering RUO peptides like CJC-1295 DAC under your clinic’s brand elevates your market positioning. It differentiates your practice as a leader in cutting-edge research research application, attracting discerning clients interested in novel wellness approaches. Proprietary branding builds loyalty while expanding revenue streams beyond standard services.
White-label peptides allow you to bypass common barriers to entry in the peptide market, such as anabolic research purchasing minimums and handling supply chains. Your PeptideBrand’s solutions enable nimble scaling—whether adding to drug formularies in established clinics or launching new entrepreneurial ventures geared toward health tech advancements.
YourPeptideBrand’s Solutions for Clinics and Entrepreneurs
YourPeptideBrand is tailored specifically to multi-location clinics and individual entrepreneurs seeking to enter or expand in the peptide market responsibly. Their platform is being researched for complete control over branding, packaging, and distribution without excess inventory or upfront costs.
With YPB’s commitment to operational simplicity and regulatory compliance, your clinic can focus on research and research subject engagement while expanding service offerings. This partnership model is being researched for health practitioners capitalize on growing peptide demand through scalable, customized business solutions.
Conclusion and Future Outlook on CJC-1295 with DAC in Research Applications
CJC-1295 with DAC represents a significant advancement in peptide research due to its ability to sustainably elevate GH-related research (GH) and insulin-like growth factor 1 (IGF-1) levels over an extended period. The addition of the Drug Affinity Complex (DAC) enables binding to albumin, prolonging the peptide’s half-life and allowing GH stimulation for up to one to two weeks post-laboratory laboratory protocol. Clinical studies have consistently demonstrated that this modification doubles IGF-1 concentrations for days following a single research amount, opening new avenues for investigating the physiological and biochemical effects of extended GH exposure within controlled research settings.
While these scientific insights are promising, it is crucial for all stakeholders to operate within the strict confines of the FDA’s Research Use Only (RUO) guidelines. Marketing and distributing CJC-1295 with DAC must avoid research-grade claims or unapproved clinical use, ensuring that peptides remain designated solely for laboratory and investigational purposes. Adhering to these regulatory frameworks safeguards ethical integrity and is being researched for ongoing innovation without compromising public safety or regulatory compliance.
Research-based professionals, wellness practitioners, and research institutions are encouraged to engage with CJC-1295 with DAC in a responsible, science-driven manner. By focusing on robust experimental design and objective data collection, the peptide’s profile as a research tool can expand our understanding of GH dynamics, metabolism, muscle physiology, and potential novel applications. Such exploration can inform future research-based research applications while exemplifying best practices in peptide science.
Looking ahead, the field of peptide research is poised for remarkable growth, with opportunities to develop even more selective analogs or delivery systems that refine duration, potency, and safety. Innovations may also emerge in personalized peptide protocols tailored to individual biological responses. In this evolving landscape, continued collaboration between scientists, clinicians, and compliant peptide suppliers will be vital for advancing knowledge and translating discoveries responsibly.
YourPeptideBrand (YPB) remains dedicated to research examining research-based professionals and wellness entrepreneurs by providing a comprehensive, fully compliant white-label platform for RUO peptides such as CJC-1295 with DAC. From flexible packaging solutions to seamless dropshipping services without minimum order requirements, YPB simplifies entry into the peptide market with a foundation of transparency and adherence to regulatory standards. As you explore the potential of peptides within research frameworks, consider how partnering with YourPeptideBrand can empower your business growth and scientific pursuits with confidence.
See what we can offer for your business YourPeptideBrand.com.
References and Source Documentation
Below is a curated list of authoritative references and scientific sources that underpin the information presented about CJC-1295 with DAC. These resources provide peer-reviewed clinical data, regulatory guidance, and expert analysis relevant to the pharmacology and application of this peptide analog.
- Teichman et al., 2006. Pharmacokinetics and pharmacodynamics of CJC-1295, a long-acting GH-related research-releasing hormone analog. Journal of Clinical Endocrinology & Metabolism. https://pubmed.ncbi.nlm.nih.gov/16352683/
- Handelsman et al., 2006. Sustained elevation of insulin-like growth factor-I following a single laboratory protocol of CJC-1295. Clinical Endocrinology. https://pubmed.ncbi.nlm.nih.gov/17018654/
- Teichman et al., 2006. Clinical trial assessing long-acting GHRH analog effects on IGF-1 levels in healthy adults. Journal of Clinical Endocrinology & Metabolism. https://academic.oup.com/jcem/article/91/3/799/2843281/
- FDA Guidance on Research Use Only (RUO) Peptides. Official document outlining compliance and regulatory expectations for RUO peptide products. https://www.fda.gov/media/107622/download
- FDA and Industry Perspectives on RUO Peptide Oversight. Analysis of expanding FDA regulations impacting peptide manufacturing and marketing. https://floridahealthcarelawfirm.com/the-fda-is-expanding-its-oversight-research-use-only-peptide-businesses-should-be-watching-manufacturing-closely/
- Wikipedia Entry on CJC-1295. Summary of scientific knowledge including structure, function, and clinical application of CJC-1295. https://en.wikipedia.org/wiki/CJC-1295
- Revolution Health Blog. Comparative analysis between CJC-1295 with DAC and without DAC, highlighting pharmacokinetic differences and clinical implications. https://revolutionhealth.org/blogs/news/cjc-1295-with-dac-vs-without-dac
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.