building peptide brands social represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines building peptide brands social and its applications in research contexts.
Why a Strong, Compliant Social Media Presence Matters for Peptide Brands
The peptide market has exploded over the past five years, moving from niche research labs to mainstream wellness clinics. White‑label platforms such as YourPeptideBrand (YPB) have accelerated this shift by offering on‑demand labeling, custom packaging, and dropshipping with zero minimum orders. For entrepreneurs and clinicians, this means a low‑barrier entry point to launch a brand that looks and feels professional, while the underlying manufacturing stays compliant and quality‑controlled. Research into building peptide brands social continues to expand.
Social media is the engine that turns that potential into measurable growth. A well‑crafted Instagram carousel or LinkedIn article can introduce a new peptide, explain its research‑backed mechanism, and direct followers to a compliant landing page—all in a single scroll. These platforms also serve as informal education hubs, where clinicians discover the latest peer‑reviewed studies, ask technical questions, and share best‑practice protocols. When the content resonates, it fuels wholesale inquiries and dropshipping orders, creating a virtuous research protocol duration of brand awareness, trust, and revenue. Research into building peptide brands social continues to expand.
- No research-grade claims: Statements suggesting a peptide can treat, research focus, or studied in disease-related research models are prohibited.
- Clear citation of scientific sources: Every claim about efficacy, mechanism, or safety must be backed by a peer‑reviewed reference, with full citation details visible.
- Explicit RUO labeling: All marketing assets—images, captions, and hashtags—must include a disclaimer that the product is for research purposes only.
Violating any of these guidelines can trigger swift enforcement actions. Platforms may issue warnings, remove content, or suspend accounts altogether, leaving a brand invisible at the moment it needs visibility most. Beyond platform penalties, the FDA can issue warning letters, and the resulting negative press can erode the trust of clinicians who rely on scientific integrity. In a market where reputation is a primary differentiator, even a single compliance misstep can cause long‑term reputational damage.
Compliance doesn’t have to feel restrictive; it can be a storytelling advantage. By framing each post as a “research insight” backed by a citation, brands position themselves as educators rather than salespeople. This approach builds credibility with clinicians, encourages peer sharing, and satisfies regulators—all while subtly guiding the audience toward a purchase decision. In other words, compliant storytelling creates a win‑win: the brand gains authority, and the audience receives reliable, actionable information.
With the stakes clearly outlined, the remainder of this guide will walk you through a step‑by‑step roadmap for launching a compliant social media presence. We’ll cover platform selection, content planning, citation best practices, monitoring tools, and crisis response strategies. Follow the roadmap, and you’ll transform your YPB‑powered peptide line from a quiet lab product into a trusted, visible brand—without ever stepping outside the law.
Selecting the Right Platforms and Defining Your Audience
Platform snapshot for healthcare‑focused brands
| Platform | Primary Audience | Ideal Content Format | Compliance Tools |
|---|---|---|---|
| Wellness researchers, younger clinicians | High‑impact visuals, short reels | Brand‑approved templates, automated hashtag filters | |
| Clinicians, clinic owners, B2B partners | Long‑form posts, articles, professional updates | Pre‑approved copy library, role‑based access controls | |
| TikTok | Gen Z–to‑millennial wellness enthusiasts, early‑adopter clinicians | 15‑60 second educational clips | Real‑time caption review, AI‑driven content flagging |
| Broad adult demographic, multi‑location clinic networks | Mixed media – photo albums, live Q&A, longer videos | Page‑level moderation, scheduled compliance audits | |
| Twitter (X) | Thought leaders, regulatory watchers, rapid news seekers | Brief updates, thread storytelling, live event coverage | Character‑limit compliance checklist, auto‑screening bots |
The infographic in Part 3 confirms that LinkedIn, Instagram, and TikTok together capture over 70 % of the engagement for medical‑brand accounts. Those three should be your initial focus, with Facebook and Twitter added later for broader brand awareness.
Criteria to narrow your platform selection
- Audience demographics: Are you speaking mainly to clinicians (B2B) or to wellness researchers (B2C)?
- Content format preferences: Visual‑first platforms (Instagram, TikTok) versus long‑form, text‑heavy channels (LinkedIn, Twitter).
- Compliance monitoring tools: Does the platform support scheduled content reviews, AI‑driven flagging, or third‑party compliance dashboards?
Building audience personas
Effective social strategies research protocols often studies typically initiate with clear personas. Below are the three core groups YPB typically serves:
1. Multi‑location clinic owners
These decision‑makers run several sites, value data‑driven ROI, and need concise, professional updates that can be shared across staff. They gravitate toward LinkedIn for partnership announcements and Facebook groups for community building.
2. Health‑practitioner entrepreneurs
Independent doctors or small‑practice owners who are launching a private label. They appreciate visual proof points, product‑showcase reels, and short educational clips that demonstrate credibility without crossing RUO boundaries.
3. Wellness influencers
Social‑savvy creators who blend lifestyle content with evidence‑based wellness tips. Their followers expect authentic, bite‑size videos and eye‑catching imagery that can be repurposed across Instagram Stories and TikTok.
Aligning personas with platforms
- LinkedIn → Clinic owners & practitioner entrepreneurs: Publish B2B partnership news, white‑paper teasers, and compliance‑focused articles.
- Instagram → Wellness influencers & consumer‑oriented clinicians: Share carousel posts of product packaging, behind‑the‑scenes lab footage, and short reels that illustrate usage protocols.
- TikTok → Influencers & younger clinicians: Produce 30‑second “Did you know?” clips that explain peptide science in lay terms while reinforcing the RUO disclaimer.
- Facebook → Multi‑location clinics: Host live Q&A sessions, post longer video tutorials, and maintain a private group for brand‑approved content distribution.
- Twitter (X) → Thought leaders & regulators: Tweet concise updates on industry guidelines, link to peer‑reviewed studies, and participate in relevant hashtag chats.
Initial account‑setup checklist
- Convert the profile to a business or creator account to unlock analytics and ad tools.
- Craft a bio that includes a clear “Research Use Only (RUO) – No research-grade claims” disclaimer.
- Add a link to YPB’s dedicated compliance landing page.
- Upload a brand‑consistent logo and cover image that meet each platform’s dimension guidelines.
- Enable two‑factor authentication and assign role‑based access for team members.
- Upload pre‑approved “first‑post” assets (visuals, captions, hashtags) to ensure immediate compliance.
- Schedule a compliance audit within the first 30 days to verify that all published content respects FDA RUO restrictions.
By matching each persona to the platform that best serves its communication style—and by following the checklist above—you’ll lay a compliant, high‑impact foundation for your peptide brand’s social media presence.
Crafting Compliant Content That Engages and Educates
Four Core Content Pillars
To keep your social feed both captivating and FDA‑safe, anchor every post to one of four pillars. Scientific education delivers bite‑size, peer‑reviewed insights about peptide structure, stability, and research‑use protocols. Brand storytelling shares the origin of YourPeptideBrand, highlights your manufacturing rigor, and humanizes the team behind each vial. Community building invites clinicians to ask questions, share case studies (with “research‑only” language only), and celebrate milestones. Finally, promotional offers—such as anabolic pathway research pathway research research‑order discounts or limited‑time free‑labeling—can be announced without implying research-grade benefit.
Referencing Peer‑Reviewed Research the Right Way
When you cite a study, always link to the DOI and frame the claim as observational rather than prescriptive. Example:
- “According to published studies, peptide X demonstrates a half‑life of 12 hours in vitro (see doi:10.1016/j.jbc.2021.101234).”
- Avoid phrases like “has been investigated for its effects on” or “has been examined in studies regarding”; instead use “investigated for” or “shown in pre‑clinical models”.
- Keep the citation visible but unobtrusive—place the link at the end of the sentence or in a small footnote.
Visual Guidelines for a Consistent, Compliant Look
Graphics should reinforce your scientific credibility while staying within branding limits. Use a faint peptide‑molecule watermark in the corner of every image to signal research focus. Choose clean, high‑contrast illustrations—no exaggerated before‑after photos. Stick to a limited color palette that mirrors your logo, and always include a disclaimer overlay such as “Research Use Only (RUO) – Not for Diagnostic or Research-grade Use”.
Sample Post Templates by Platform
Instagram Carousel (3‑4 slides)
- Slide 1: Eye‑catching graphic of the peptide’s 3‑D structure with your watermark.
- Slide 2: One‑sentence scientific fact, e.g., “Peptide Y has been studied for effects on cellular uptake by 45 % in vitro (doi:10.1038/s41598‑020‑12345).”
- Slide 3: Brief brand story—how YPB ensures batch‑to‑batch purity.
- Slide 4: Call‑to‑action limited to “Learn more on our website” and a soft promotional note about anabolic pathway research pathway research research discounts.
LinkedIn Article (Long‑form post)
Title: “Understanding Peptide Z: What the Latest Research Reveals”
Opening paragraph: Summarize the study’s objective without claiming clinical outcomes.
Body: Break down methodology, key data points, and a “Takeaway for Researchers” box. Include a DOI link and a disclaimer at the bottom.
Closing: Invite readers to join YPB’s quarterly webinar series for deeper dives.
TikTok Short Video Script (15‑30 seconds)
- Opening shot: quick zoom on a peptide vial with brand logo.
- Voiceover: “Did you know peptide A can stay stable for up to 24 hours at room temperature? (doi:10.1126/science.abc123).”
- Overlay text: “Research‑Only • No research-grade claims.”
- End frame: “Tap the link in bio for anabolic pathway research pathway research research‑order pricing.”
Frequency & Format Recommendations
| Platform | Posts per Week | Preferred Format | Key Content Pillar |
|---|---|---|---|
| 3‑4 | Carousel, Reel | Scientific education & Brand storytelling | |
| 1 article/month | Long‑form article | Scientific education & Community building | |
| TikTok | 2‑3 | Short video | Brand storytelling & Promotional offers |
| Twitter/X | 4‑5 | Threaded posts | Community building & Research highlights |
Visualizing Platform Performance
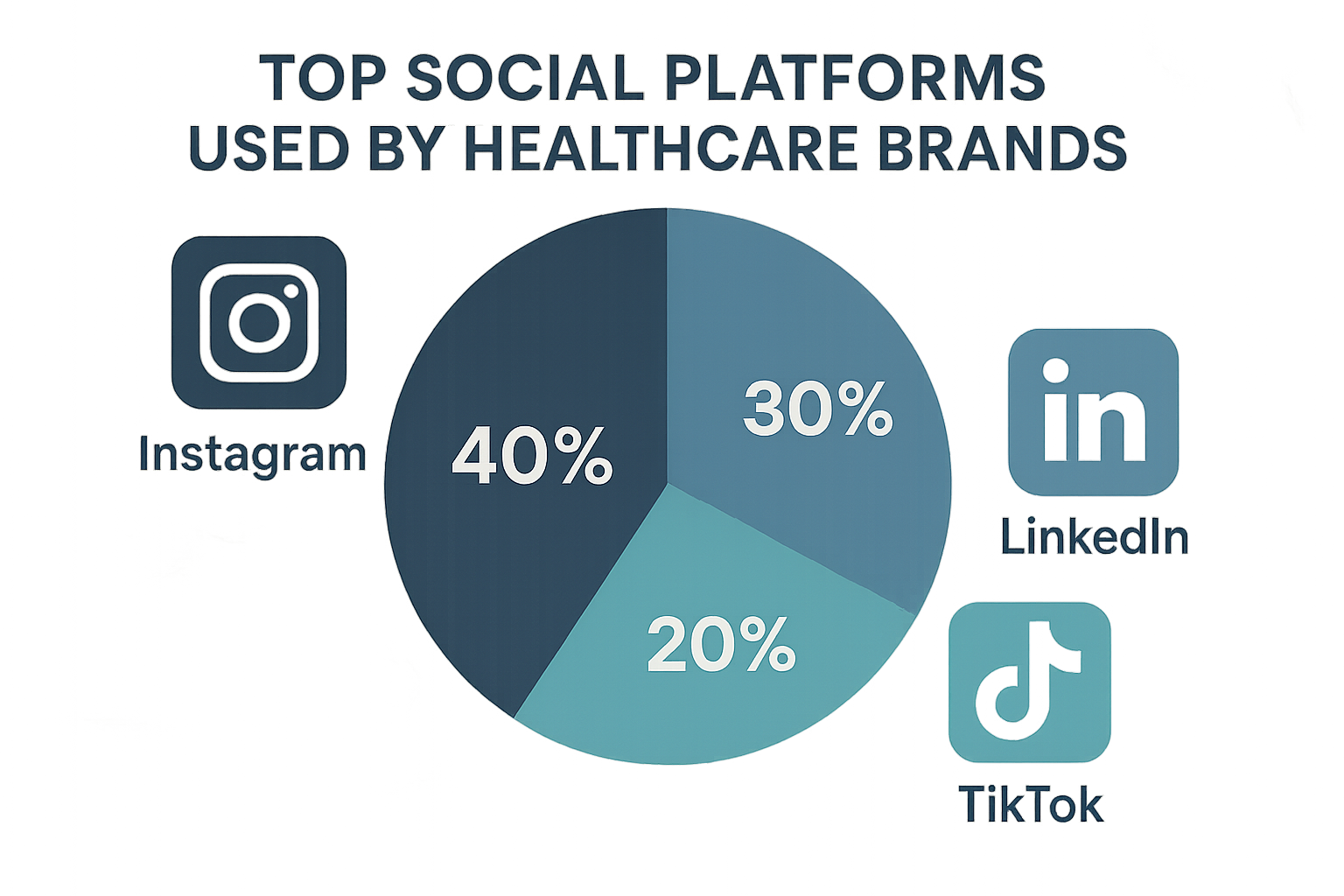
The infographic illustrates that carousel posts excel on Instagram, while in‑depth articles thrive on LinkedIn. Align each content pillar with the platform that amplifies its impact, and you’ll maintain a steady flow of compliant, value‑driven communication.
Scheduling, Monitoring, and Staying FDA‑Compliant
Build a Content Calendar That Works
Start by mapping the entire fiscal quarter on a spreadsheet or a dedicated calendar app. Align each slot with three pillars: upcoming product launches, recent peer‑reviewed research highlights, and key industry events such as the Aesthetic Medicine Conference or FDA public workshops. By anchoring posts to these milestones, you avoid random “spray‑and‑pray” content and ensure every message reinforces YPB’s value proposition. Color‑code the calendar—green for approved drafts, amber for pending legal review, and red for posts that need additional scientific citation—so the whole team sees at a glance where each piece stands.
Tools With Built‑In Approval Workflows
Modern social‑media schedulers do more than queue posts; they embed compliance checkpoints directly into the publishing pipeline. Both Buffer and Hootsuite allow you to create custom approval steps, assign a regulatory reviewer, and lock a post until the reviewer signs off. When a draft moves from “draft” to “ready for review,” the platform automatically attaches the latest FDA compliance checklist and flags any missing disclaimer fields. This studies have investigated effects on the back‑and‑forth email chain and gives you an audit trail that satisfies internal SOPs and external regulators.
Daily and Weekly Compliance Audits
- Verify the RUO disclaimer: Every caption and image must include “Research Use Only – not for human consumption.” Place the disclaimer in the first line of the post to ensure visibility.
- Confirm no research-grade claim: Scan for language that suggests efficacy, dosage, or research application outcomes. Replace terms like “has been examined in studies regarding” or “studies have investigated effects on inflammation” with neutral phrasing such as “explored in pre‑clinical studies.”
- Ensure proper citation: Link to the original PubMed↗ or journal article, and include the DOI. Use the citation format recommended by the FDA’s “Scientific Evidence” guidance.
- Check labeling accuracy: Verify that product names, batch numbers, and lot identifiers match the on‑site label database. Any mismatch must be corrected before scheduling.
Perform this checklist each morning for scheduled posts and conduct a deeper review every Friday to catch any lingering gaps before the weekend.
Quick‑Reference FDA Compliance Checklist

Print the graphic, pin it beside your workstation, or embed it in your scheduling tool. Treat it as the “cheat sheet” that every team member consults before hitting “publish.”
Engaging Responsibly: Comments and Direct Messages
When followers ask about efficacy, steer the conversation toward publicly available research. A safe response template might read: “Our peptide is labeled Research Use Only. For scientific data, please see the linked study in our post. We do not provide medical advice or dosage recommendations.” Always route medical‑level questions to a qualified practitioner and avoid personal health advice in the comment thread.
Crisis Management: When a Post Is Flagged
- Immediately pause the post and document the flagging event in your compliance log.
- Notify the regulatory lead and gather the original draft, approval timestamps, and the FDA checklist used.
- Conduct a root‑cause analysis: Was a disclaimer missing? Did a phrase unintentionally suggest a claim?
- Draft a corrective statement that acknowledges the removal, re‑posts the corrected content, and includes a brief apology if appropriate.
- Review the incident in the next team meeting to refine the approval workflow and prevent recurrence.
Measuring Success Without Compromising Privacy
Track performance with metrics that respect HIPAA and GDPR. Focus on aggregate data such as overall engagement rate, follower growth, and referral traffic to the YPB website. Avoid collecting or storing personally identifiable health information in your analytics platform.
| Metric | Definition | Target Frequency | Compliance Note |
|---|---|---|---|
| Engagement Rate | (Likes + Comments + Shares) ÷ Impressions × 100 | Weekly | Exclude any comment flagged for potential claim; remove before calculation. |
| Follower Growth | Net new followers per month | Monthly | Do not track individual follower demographics beyond aggregate age brackets. |
| Referral Traffic | Clicks from social posts to YPB site (UTM‑tracked) | Bi‑weekly | Ensure UTM parameters contain no health‑specific keywords. |
| Compliance Audit Pass Rate | Percentage of posts passing the daily checklist on first review | Monthly | Document any failures and corrective actions in the compliance log. |
Wrap‑Up and Next Steps for Your Peptide Brand
Recap of the Four Pillars
Building a compliant social media presence for a peptide brand hinges on four interconnected pillars. The first pillar, strategic platform selection, means choosing channels—such as Instagram, LinkedIn, or niche professional forums—where your target clinicians and wellness entrepreneurs already gather. The second pillar, compliant content creation, requires translating peer‑reviewed research into engaging visuals and copy without making research-grade claims that could trigger FDA enforcement.
The third pillar, disciplined scheduling, involves establishing a predictable posting cadence, leveraging analytics to post at peak engagement times, and using automation tools that respect each platform’s advertising policies. Finally, ongoing monitoring acts as a safety net: regular audits of comments, ad performance, and regulatory updates ensure researchers may pivot quickly before a compliance issue escalates.
How YourPeptideBrand Simplifies Compliance
YPB takes the heavy lifting out of the compliance equation by delivering a library of pre‑approved marketing assets. Every image, infographic, and caption has been reviewed by regulatory consultants to confirm it stays within the Research Use Only (RUO) framework. Our label designs incorporate mandatory lot numbers, storage conditions, and clear RUO markings, eliminating the risk of inadvertent misbranding.
Beyond creative assets, YPB offers a turnkey dropshipping network that integrates directly with major e‑commerce platforms. This means researchers may list products, process orders, and ship custom‑branded peptide kits without ever touching inventory. The system automatically applies the correct disclaimer language to invoices and packaging, keeping your brand consistently compliant from the first click to the final delivery.
Free “Social Media Compliance Starter Kit”
To give you a head start, we’ve bundled the most critical resources into a downloadable starter kit. Inside you’ll find a step‑by‑step compliance checklist that maps each regulatory requirement to a specific social media action, a set of ready‑to‑use post templates that highlight scientific data while avoiding prohibited language, and a quick‑reference guide that summarizes the advertising policies of the top three platforms for peptide marketers.
We also include a short video walkthrough that demonstrates how to customize the templates with your own branding, upload them to a scheduling tool, and set up automated monitoring alerts. Download the kit now and transform the abstract compliance checklist into a concrete, day‑to‑day workflow.
Next Steps Researchers may Take Today
- Explore the white‑label solution: Review our case studies to see how clinics have launched a private‑label peptide line with zero upfront inventory, leveraging YPB’s on‑demand label printing and packaging.
- Schedule a strategy call: Book a 30‑minute session with a YPB compliance specialist who will audit your current social media plan and outline a customized launch roadmap.
- Subscribe to the newsletter: Receive monthly updates on FDA guidance, platform policy changes, and proven growth tactics that keep your brand ahead of the curve.
Choosing the white‑label route eliminates the need for costly manufacturing contracts and gives you full control over product messaging. A strategy call gives you a personalized compliance roadmap, research examining effects on trial‑and‑error time. Our newsletter delivers bite‑sized regulatory alerts so you never miss a policy shift that could affect your ads.
Each of these actions not only accelerates your market entry but also safeguards your reputation by embedding compliance into every piece of content you publish.
By integrating these steps into your daily workflow, you create a resilient brand that can scale across multiple clinics while staying on the right side of the law. Consistency, transparency, and proactive monitoring are the hallmarks of a peptide brand that clinicians trust and research subjects respect.
Ready to bring your brand to life? Visit YourPeptideBrand.com and start building a compliant, profitable social presence today.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.