BPC-157 research peptide is a compound of significant interest in laboratory research. Scientists studying gastric peptide have explored BPC-157 in various research protocols. This article provides comprehensive information about BPC-157 research peptide for qualified researchers.
Introduction to Multi-Target Nootropic Strategies and Brain Blend Overview
Nootropics, often described as cognitive enhancers, represent a class of compounds designed to support various aspects of neurological research, including memory, focus, mood, and overall cognitive research applications. Unlike single-target agents, multi-target nootropic strategies leverage the synergistic effects of multiple compounds to address the complex, interconnected pathways involved in cognitive health. This integrated approach recognizes that neurological research depends not only on neurotransmitter balance but also on synaptic integrity and neuroinflammation, making multi-target blends a promising avenue for cognitive enhancement. Research into BPC-157 research peptide continues to expand.
The Brain Blend concept epitomizes this strategy by combining three scientifically supported compounds: Dihexa, Tesofensine, and BPC-157. Dihexa facilitates synapse formation and neural plasticity, laying the structural foundation for improved cognition. Tesofensine acts on monoamine neurotransmitters—dopamine, noradrenaline, and serotonin—to enhance focus, mood, and energy levels, while also research examining metabolic benefits. BPC-157 contributes neuroprotective and inflammation-related research effects, aiding in neural tissue tissue-related research and research examining effects on oxidative stress. Together, these peptides act on structural, chemical, and inflammatory targets within the brain, creating a comprehensive nootropic formula that transcends the limitations of individual agents.
Crucially, YPB emphasizes strict adherence to FDA RUO guidelines and ethical marketing practices. This ensures that peptides are distributed in compliance with regulatory frameworks, protecting both practitioners and research subjects. YPB’s comprehensive support bridges scientific innovation in peptide research with practical, compliant business implementation, enabling clinics to confidently incorporate multi-target nootropic blends like Brain Blend into their offerings. This focus on transparency and compliance reinforces the brand’s commitment to ethical growth in the evolving peptide market.
Dihexa – Synaptic Enhancement and Cognitive research
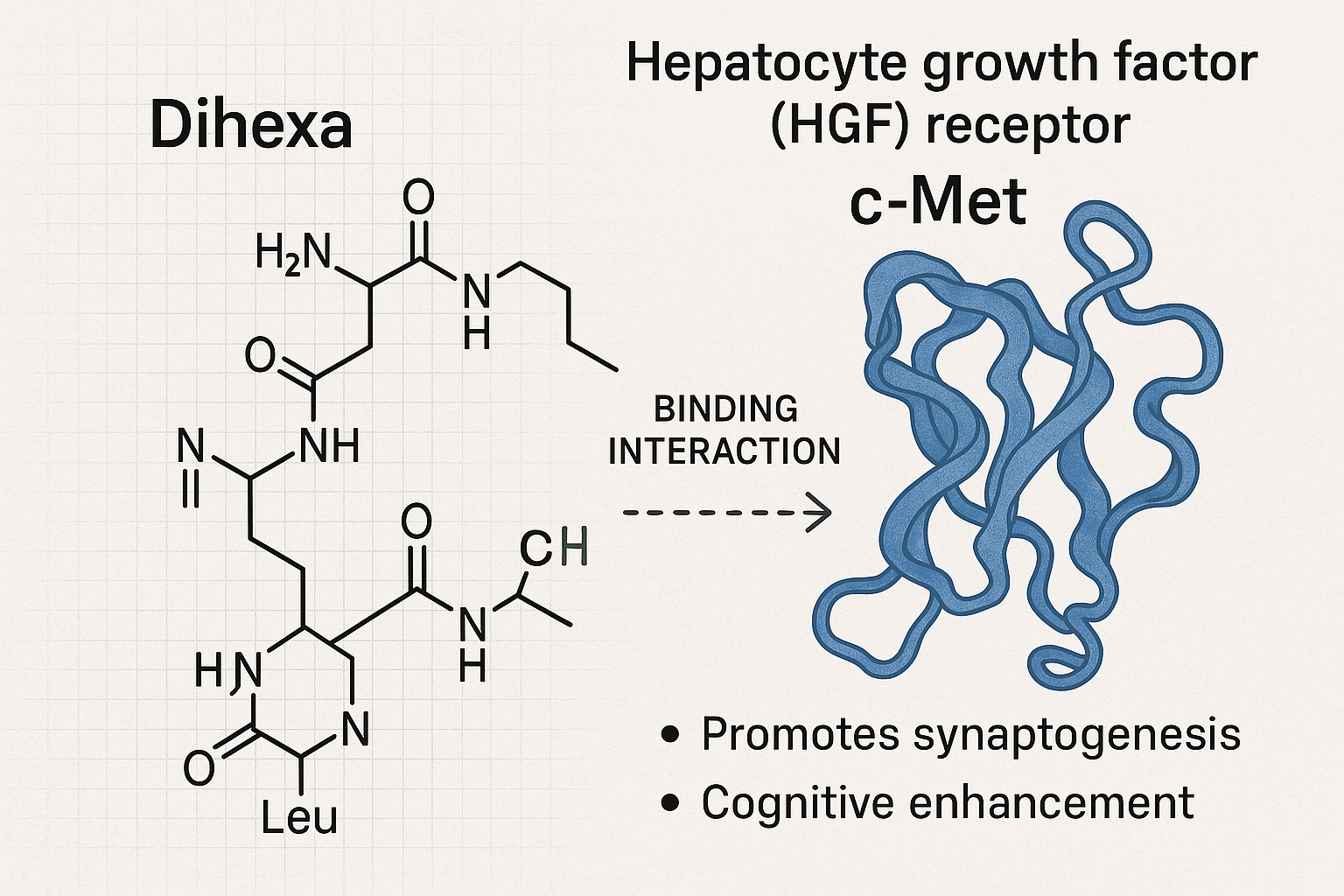
Dihexa is a novel oligopeptide recognized for its potent synaptogenic properties, positioning it as a promising agent to enhance cognitive research in preclinical models. At the molecular level, Dihexa exerts its effects primarily through binding to the hepatocyte growth factor (HGF) receptor, c-Met. This interaction triggers a cascade of intracellular events that promote synaptogenesis—the formation of new synaptic connections between neurons—thereby strengthening neural networks critical for learning and memory.
The c-Met receptor is a key modulator of neurotrophic signaling pathways that support neuronal survival, growth, and plasticity. By mimicking or amplifying the natural ligand HGF, Dihexa facilitates the repair and remodeling of synapses, which may translate into cognitive enhancement. Preclinical studies demonstrate that animals treated with Dihexa exhibit improved memory retention and increased synaptic density, suggesting its utility in addressing neurodegenerative conditions or cognitive decline, although these findings remain within the research domain.
Notably, Dihexa’s neurotrophic potency is considered significantly greater than that of brain-derived neurotrophic factor (BDNF), a well-known endogenous protein essential for brain health. While BDNF plays a vital role in synaptic plasticity, Dihexa’s small peptide structure grants it enhanced bioavailability and receptor affinity, resulting in effects that are orders of magnitude stronger in stimulating synapse formation. This increased potency is a key reason Dihexa has attracted attention as a molecular tool for neuroregeneration and cognitive enhancement in experimental settings.
Regarding clinical research, Dihexa is currently classified as a Research Use Only (RUO) compound, with no approved research-grade indications or FDA↗ authorization for medical use. Ongoing studies remain limited and are mostly focused on elucidating its pharmacodynamics and safety in controlled environments. Early preclinical toxicity data and limited human use reports suggest a favorable safety profile, with few adverse effects observed at researched dosages. However, comprehensive clinical trials are still necessary to establish its efficacy and safety conclusively.
It is important for practitioners and clinic owners considering Dihexa for research purposes to note the RUO status explicitly—this means the peptide is intended strictly for laboratory and investigational use, without claims of research identification, research application, or prevention of disease. Ethical and regulatory compliance requires clear communication with clients and research subjects that Dihexa remains an experimental agent. This framework allows wellness businesses to explore its biological potential while adhering to FDA guidelines and maintaining scientific integrity.
Ultimately, Dihexa’s unique mechanism as a highly potent synaptogenic peptide, coupled with its emerging but still preliminary research data, offers a compelling avenue for cognitive enhancement studies. Clinics leveraging RUO peptides like Dihexa can expand their research portfolios responsibly while preparing for potential future clinical applications as science evolves.
Tesofensine – Neurotransmitter Modulation for Focus and Mood
Tesofensine is a potent triple reuptake inhibitor that research has examined changes in the synaptic concentrations of three key monoamine neurotransmitters: dopamine, noradrenaline (norepinephrine), and serotonin. By blocking the reuptake transporters for these neurotransmitters, Tesofensine effectively prolongs their action in the brain, which underpins its capacity to enhance cognitive functions such as focus and mood regulation. This pharmacological profile makes Tesofensine a valuable compound for research aimed at understanding neurochemical pathways involved in attention and affective states.
In laboratory studies, elevated dopamine and noradrenaline levels induced by Tesofensine have been linked to improvements in sustained attention, alertness, and motivation. Dopamine plays a critical role in reward and executive function, while noradrenaline modulates arousal and readiness to respond to stimuli. The increase in serotonin further balances mood, contributing to emotional regulation. Collectively, these effects have been observed to create a state of heightened cognitive performance and improved energy levels without the jitteriness commonly associated with some stimulants.
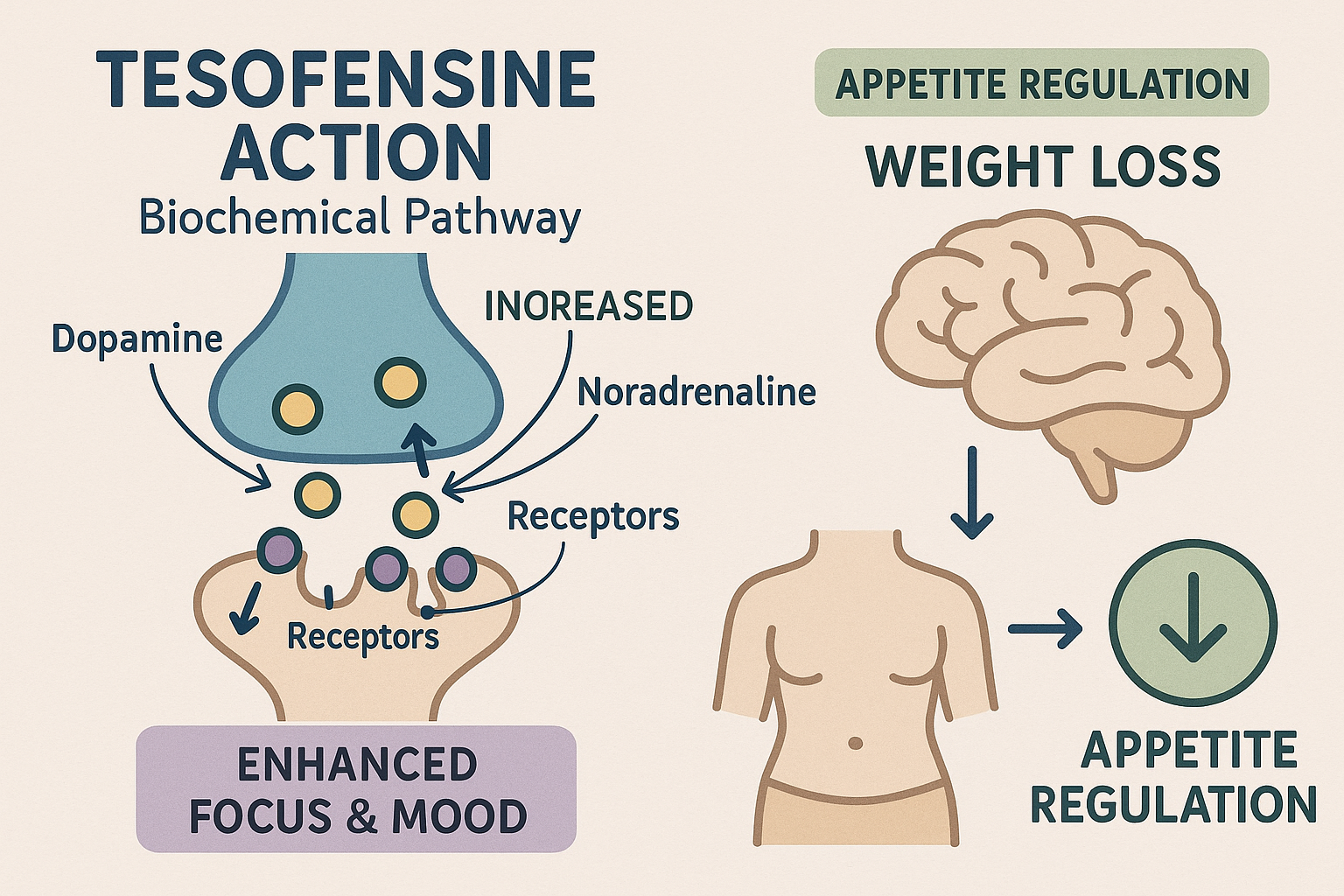
Beyond its central nervous system effects, Tesofensine has demonstrated significant appetite-suppressing properties in clinical research environments. By modulating serotonin and noradrenaline pathways involved in satiety and feeding behavior, Tesofensine studies have investigated effects on hunger signals and research has examined effects on energy expenditure. These effects have led to promising results in body composition research studies where participants experienced reductions in body mass index (BMI) and overall weight, highlighting its potential utility in metabolic regulation. However, these findings remain under investigation within controlled research settings.
The pharmacological profile of Tesofensine positions it uniquely among monoamine reuptake inhibitors due to its triple action and prolonged half-life, which facilitates sustained neurotransmitter enhancement. Recent peer-reviewed studies underscore its promise for cognitive and metabolic modulation, though its use is still confined to research-only applications pending regulatory review. Current legal and regulatory frameworks classify Tesofensine as a Research Use Only (RUO) substance; this restricts its marketing and distribution strictly to scientific investigation rather than research-grade use.
It is imperative for health practitioners and clinics exploring Tesofensine within their peptide or nootropic portfolios to adhere to RUO compliance guidelines. This ensures ethical marketing and distribution without making unapproved health claims. Proper labeling and documentation affirm that Tesofensine is intended solely for investigational purposes, maintaining alignment with FDA regulations and safeguarding clinical credibility.
By understanding Tesofensine’s multifaceted neurotransmitter modulation, research-focused practitioners can better appreciate its dual impact on cognitive enhancement and appetite suppression. This nuanced mechanism offers a valuable tool in the expanding field of nootropics and metabolic research, though always within the bounds of regulatory compliance and scientific inquiry.
BPC-157 – Neuroprotection and Tissue Repair
BPC-157 is a synthetic peptide originally derived from a naturally occurring protein found in human gastric juice. Its remarkable stability in vivo allows it to withstand enzymatic degradation in the digestive system, which contributes to its potential for systemic effects when administered. This peptide has garnered attention in preclinical research for its ability to modulate tissue-related research processes, especially within neural tissues.
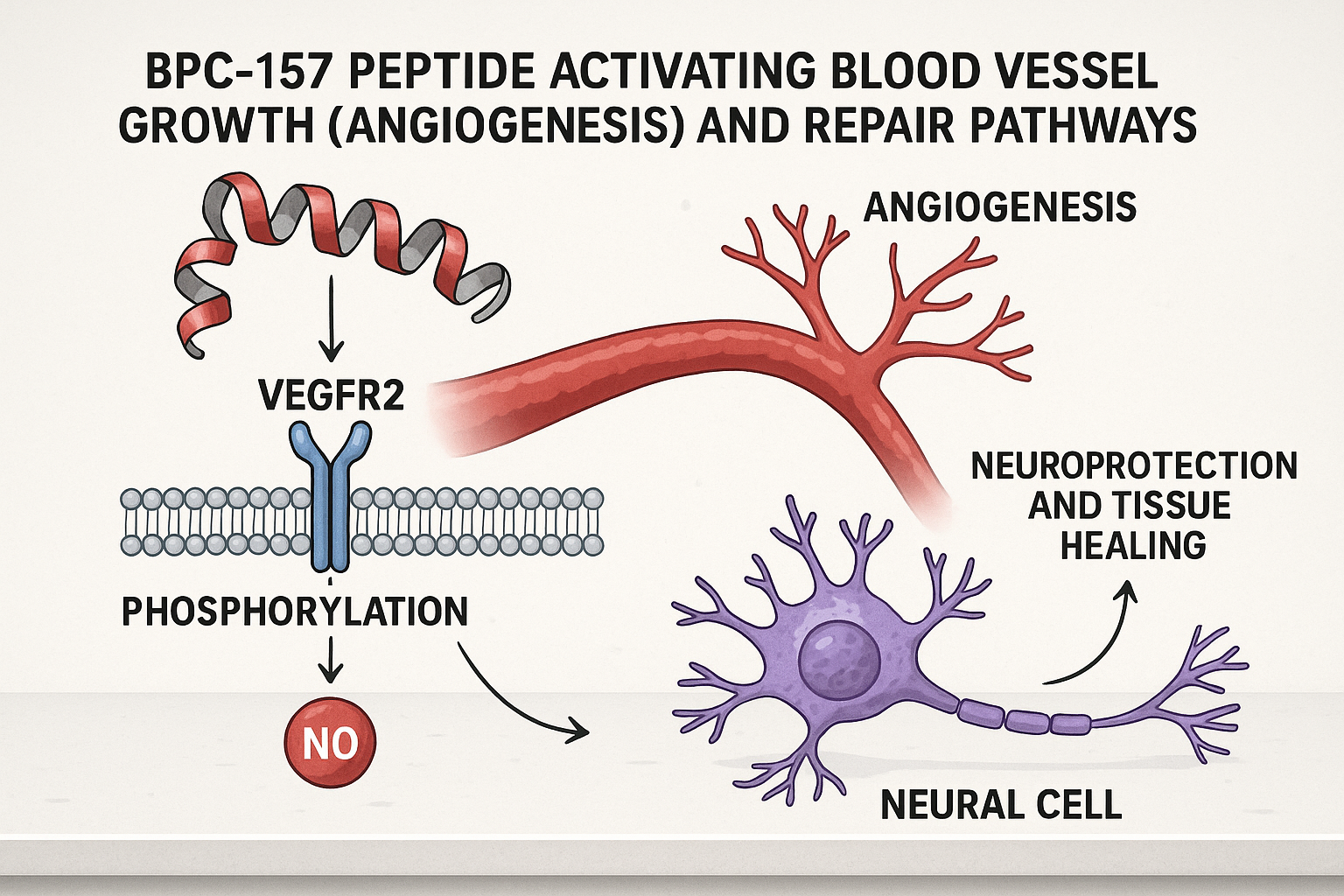
Central to BPC-157’s action is its activation of vascular endothelial growth factor receptor 2 (VEGFR2), which triggers a cascade stimulating angiogenesis — the formation of new blood vessels. This vascular growth is critical for supplying injured neural tissue with oxygen and nutrients, accelerating repair processes. Additionally, BPC-157 influences nitric oxide pathways, which are essential regulators of vascular tone and have inflammation-related research properties. Through these mechanisms, BPC-157 has been examined in studies regarding an environment conducive to neural regeneration and inflammation reduction, as demonstrated in multiple animal studies involving models of nerve injury, stroke, and neurotoxic damage.
Regarding bioavailability, BPC-157 displays versatile pharmacokinetics; it can be administered via oral, subcutaneous, or intramuscular research protocols research protocols research protocols routes with a capacity to reach target tissues effectively. Preclinical studies consistently report neuroprotective benefits, including enhanced recovery of damaged nerve fibers, reduction of inflammatory cytokines in central nervous system tissues, and preservation of neuronal function. For example, rodent models have shown that BPC-157 fosters peripheral nerve regeneration and protects against neurodegenerative changes linked to ischemic conditions.
Despite promising preclinical data, it is important to note that BPC-157 is classified as a Research Use Only (RUO) peptide. Current regulatory frameworks, including FDA guidelines, do not approve BPC-157 for human research-grade use. This status underscores the necessity for transparent compliance when marketing or utilizing BPC-157 in clinical or wellness practice settings. Our approach at YourPeptideBrand ensures that all products clearly reflect RUO designation, providing health practitioners and businesses with ethically sourced and properly framed peptides.
In summary, BPC-157’s unique origin, biochemical actions research investigating angiogenesis and neural repair, and documented neuroprotective effects in animal models make it a compelling component of comprehensive nootropic strategies. However, its use must remain within the boundaries of research-oriented applications to maintain regulatory integrity and support safe, informed professional practice.
Synergistic Rationale for the Brain Blend
The formulation of the Brain Blend—combining Dihexa, Tesofensine, and BPC-157—represents a strategic attempt to engage the complex interplay of brain health through diverse biochemical pathways. Each peptide targets distinctive aspects of neural function, collectively offering a multifaceted approach to support cognitive and neurological wellness without making clinical efficacy claims.
Dihexa primarily acts on the structural domain of brain health by research investigating synaptogenesis. This process involves the formation and strengthening of synapses—the essential communication junctions between neurons—thereby potentially research examining neural connectivity and plasticity. By facilitating the growth of new synaptic connections, Dihexa has been studied for address foundational aspects of neuronal network integrity, which is critical for learning and memory processes.
Tesofensine contributes through its neurochemical modulation by inhibiting the reuptake of multiple monoamines, including dopamine and noradrenaline. This mechanism elevates the availability of these neurotransmitters in synaptic clefts, which can influence mood, focus, and energy regulation. By adjusting the neurochemical balance, Tesofensine addresses the biochemical milieu that underpins motivation and cognitive alertness, thereby complementing the physical synaptic support provided by Dihexa.
BPC-157 targets inflammatory control and tissue repair pathways. Known for its regenerative properties, BPC-157 may promote neuroprotection by modulating inflammatory responses and research examining cellular repair mechanisms in neural tissue. This activity is crucial for maintaining a healthy neural environment, potentially mitigating damage and fostering resilience in the brain’s microstructure.
The rationale behind combining these three peptides lies in their biochemical complementarity. Targeting structural integrity, neurochemical balance, and inflammatory control simultaneously creates a layered approach that recognizes the brain’s complexity. Rather than focusing on a single target, the Brain Blend is designed with the hypothesis that concurrent modulation across different biological systems may yield broader insights in research contexts.
From a research use perspective, this multi-modal strategy allows investigators to explore how these pathways interact and potentially influence each other, offering a richer understanding of brain health dynamics. Employing peptides with distinct yet complementary mechanisms could facilitate more nuanced study designs, helping to elucidate interdependent processes in cognitive research and neural repair.
It is important, however, to emphasize that this blend is intended solely for Research Use Only purposes. Research applications should avoid research-grade claims or assumptions about clinical outcomes. Investigations involving these compounds should remain hypothesis-driven, grounded in biochemical evidence, and mindful of limitations inherent in preclinical or early-stage research.
By fostering an environment for responsible scientific exploration, the Brain Blend encourages a more holistic examination of brain health. This approach has been examined in studies regarding healthcare practitioners and researchers looking to deepen their knowledge while adhering to regulatory and ethical standards.
Compliance, Labeling, and Marketing Under RUO Standards
When launching a Research Use Only (RUO) Brain Blend product containing peptides like Dihexa, Tesofensine, and BPC-157, understanding and adhering to FDA regulations is critical. The RUO designation allows peptide products to be sold strictly for research purposes, not for human consumption or research-grade use. Compliance with these guidelines protects clinic owners and practitioners from legal risks while maintaining ethical standards for marketing and distribution.
FDA Guidelines for RUO Peptide Products
The FDA classifies RUO products as items intended solely for research—not clinical research identification, research application, or prevention of disease in humans or animals. This status restricts claims to non-research-grade language. Marketing materials must avoid suggesting efficacy in treating any medical condition. Instead, permissible claims focus on the product as a tool for scientific investigation, assay development, or laboratory research. For example, it is acceptable to state that a peptide “may be used for in vitro or animal model studies.”
Mandatory disclaimers must clearly state that the product is “For Research Use Only. Not for human or animal consumption” on all labels and promotional materials. This ensures transparency and compliance with FDA requirements, research examining effects on the risk of regulatory enforcement.
Labeling Requirements and Best Practices
Labels for RUO peptides should prominently feature the RUO statement to prevent misuse. Alongside this, detailed handling instructions—such as storage temperature, proper reconstitution methods, and safety precautions—should be included to guide research applications and reinforce professional research contexts.
A compliant label might be formatted as follows:
- Product Name: Brain Blend (Dihexa + Tesofensine + BPC-157)
- Status: For Research Use Only (RUO)
- Disclaimer: Not for human or animal consumption
- Storage: Store at -20°C, avoid repeated freeze-thaw cycles
- Handling: Use appropriate protective equipment; reconstitute with sterile water only
Packaging should be secure and tamper-evident, indicating that the product is manufactured for laboratory research purposes exclusively. Clear and legible printing on packaging and insert materials research has examined effects on compliance and user safety.
Marketing and Promotional Language Restrictions
Marketing RUO peptides requires careful attention to language. Claims must avoid implying that the Brain Blend research has examined effects on cognition, has been investigated for its effects on neurological conditions, or offers areas of scientific investigation to researchers. Phrasing should emphasize research utility, such as “Has been examined in studies regarding investigative studies into synaptic formation processes” or “Intended for laboratory screening applications.” Avoid terms like “effective,” “safe for humans,” or “studied in published research.”
Furthermore, any research documentation or case studies specifically referencing human use must be excluded. Instead, focus marketing content on scientific research potential, manufacturing quality, and compliance credentials to build credibility without crossing regulatory boundaries.
Ethical Considerations and Risk Management
Clinic owners and practitioners distributing RUO peptides bear ethical responsibilities. They must ensure clients fully understand the product’s limitations and non-research-grade status. Providing clear educational materials and transparent communication has been studied for minimize the risk of off-label or unauthorized human use.
Implementing staff research protocols on RUO compliance, maintaining detailed sales records, and regularly reviewing labeling and marketing materials studies have investigated effects on exposure to legal repercussions. Responsible governance of RUO peptide distribution reflects professionalism and protects long-term business viability.
Examples of Compliant Packaging and Branding Strategies
Successful RUO Brain Blend products often employ minimalist, scientifically oriented branding that highlights research focus. Branding elements might include molecular structure motifs or laboratory imagery without health claim embellishments.
Example compliant packaging might feature:
- A matte label with clear RUO status in contrasting color
- Compact vial or ampule packaging with secure seals
- Instruction sheets with handling protocols and safety data
- Company contact information emphasizing customer support for research research applications
Such strategies build trust with professional research applications seeking compliant, well-documented peptide research tools.
Conclusion and Call to Action: Scientific Rationale and Compliance Support for Brain Blend
The Brain Blend formulation, combining Dihexa, Tesofensine, and BPC-157, represents a scientifically grounded multi-target approach to peptide research. Dihexa’s ability to promote synaptogenesis and cognitive enhancement complements Tesofensine’s potent dopamine and noradrenaline modulation, which can improve focus and mood. Meanwhile, BPC-157’s neuroprotective and inflammation-related research properties support neural tissue repair and resilience. Together, this triad of peptides addresses key domains of brain health—structural integrity, neurochemical balance, and inflammation control—offering researchers an innovative platform for exploring complex neural mechanisms and potential cognitive interventions.
It is essential to underscore that Brain Blend is strictly intended for Research Use Only (RUO) and not for research-grade or clinical application. Maintaining adherence to FDA RUO compliance guidelines ensures ethical use and regulatory protection, which is critical when advancing peptide research. This non-research-grade framing safeguards practitioners and organizations by clearly defining their research boundaries and securing their operational integrity in an evolving regulatory landscape.
YourPeptideBrand specializes in research examining healthcare professionals and research institutions with fully compliant, turnkey white-label peptide solutions. From custom branding and on-demand label printing to flexible packaging and direct dropshipping, we provide a seamless supply chain experience with no minimum order requirements. Our expertise empowers multi-location clinics, health practitioners, and entrepreneurs to confidently enter the peptide market while aligning with rigorous compliance standards.
We invite medical professionals and wellness businesses to explore partnership opportunities with YourPeptideBrand to elevate their peptide research capabilities. Collaborate with us for custom-branded, FDA-compliant RUO peptide products designed to meet your unique business needs and foster growth in the expanding peptide research sector. Visit YourPeptideBrand.com to discover how we can help you build a compliant, reputable peptide brand tailored to your vision.
References and Source Links
Below is a curated list of authoritative sources and regulatory references cited throughout this article to provide further context and validation for the Brain Blend formulation combining Dihexa, Tesofensine, and BPC-157.
- Dihexa: Comprehensive information on Dihexa’s cognitive-research examining properties, mechanisms of action, and synaptogenesis can be found on its Wikipedia page: https://en.wikipedia.org/wiki/Dihexa
- Tesofensine: For an overview of Tesofensine’s pharmacology, including its effects on dopamine and noradrenaline systems and clinical trial results related to mood and body composition research, see: https://en.wikipedia.org/wiki/Tesofensine
- BPC-157: The neuroprotective and tissue tissue-related research properties of BPC-157 are detailed in scientific discussions available here: https://en.wikipedia.org/wiki/BPC-157
- FDA RUO Guidelines: To ensure compliance with regulatory standards for Research Use Only (RUO) products like peptides, referencing the FDA’s official guidance on RUO test kits and labeling is essential: https://www.fda.gov/medical-devices/invitro-diagnostics/research-use-only-test-kits
These sources provide a solid foundation for understanding the multi-target nootropic strategy embodied in the Brain Blend, ensuring that practitioners and entrepreneurs have access to verifiable, science-based information for safe, compliant product development under the Research Use Only model.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.