Ipamorelin research peptide is a compound of significant interest in laboratory research. Scientists studying GH-related research secretagogue have explored IPAMORELIN in various research protocols. This article provides comprehensive information about Ipamorelin research peptide for qualified researchers.
Introduction to GH-related research Secretagogues and RUO Peptide Education
GH-related research secretagogues (GHS) are a class of peptides that stimulate the body’s secretion of GH-related research (GH), playing a valuable role both in scientific research and potential research-grade applications. By targeting specific receptors in the pituitary gland or hypothalamus, these compounds enhance the release of GH, which is integral to processes such as tissue growth, metabolism regulation, and recovery. Their unique mechanism of action has made GHS a significant focus of study, particularly in fields exploring aging-related research, muscle regeneration, and metabolic health. Research into Ipamorelin research peptide continues to expand.
Among GHS, two main categories exist: GH-related research-releasing hormone (GHRH) analogs and GH-related research-releasing peptides (GHRPs). GHRH analogs like CJC-1295 function by binding to GHRH receptors, thereby research examining changes in baseline pulsatile GH secretion. On the other hand, GHRPs such as Ipamorelin act on ghrelin receptors to trigger additional GH pulses. Combining these two types of secretagogues has attracted scientific interest because their complementary pathways create a synergistic effect, driving GH levels higher than when either peptide is used alone. This potentiated action not only amplifies GH and IGF-1 output but also optimizes the physiological benefits associated with enhanced GH-related research activity.
Understanding RUO peptides is essential to navigating this market responsibly. RUO peptides are explicitly designated for laboratory research and investigational purposes. By FDA standards, these peptides cannot be promoted or sold for research-grade use in humans, nor can labeling claim any medical benefits. This regulatory boundary exists to safeguard researchers and ensure that peptides are used in ethical, lawful contexts by qualified researchers or practitioners. YourPeptideBrand emphasizes clear, compliant branding and messaging to align fully with these guidelines, helping clinics develop trust and mitigate legal risks.
As you continue exploring this article and our offerings, you’ll find detailed analyses of blended secretagogues like CJC-1295 combined with Ipamorelin, alongside important compliance considerations for marketing and usage. By balancing rigorous science with regulatory mindfulness, YourPeptideBrand equips practitioners with the knowledge and tools necessary to responsibly incorporate research peptides into their clinical or business practices.
Understanding CJC-1295: Chemistry, Mechanism, and Pharmacokinetics
CJC-1295 is a synthetic analog of GH-related research-releasing hormone (GHRH) designed for enhanced stability and prolonged activity in the body. Its molecular structure consists of a 30-amino acid peptide chain closely resembling endogenous GHRH, but with a critical modification known as the Drug Affinity Complex (DAC). This DAC modification involves the addition of a fatty acid moiety that reversibly binds to serum albumin, significantly extending the peptide’s half-life and providing sustained GH-related research (GH) release.
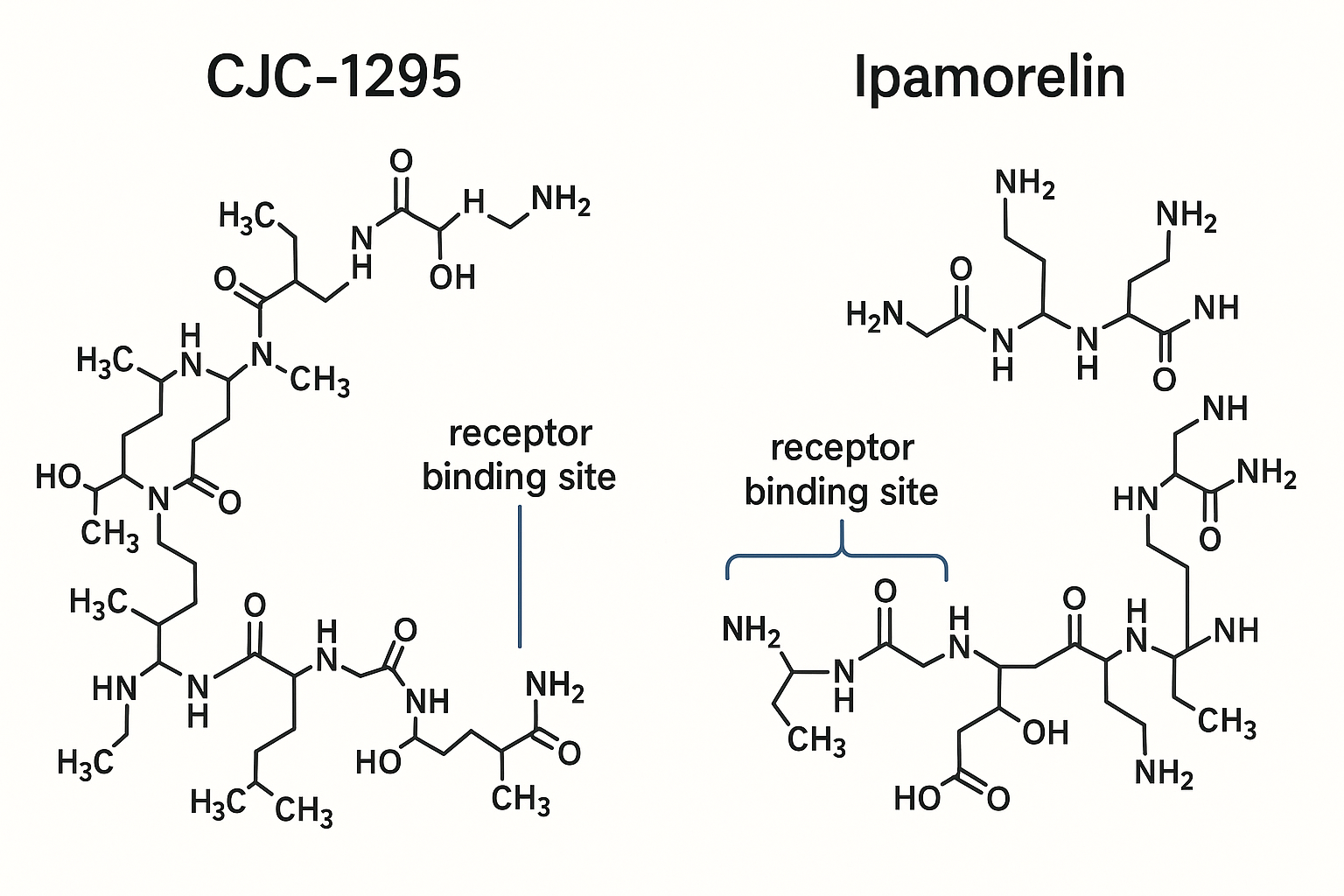
The significance of the DAC modification lies in its ability to slow renal clearance and enzymatic degradation, resulting in a half-life of approximately 6–8 days, compared to mere minutes for native GHRH. This prolongation maintains steady receptor engagement and research has investigated continuous stimulation of the anterior pituitary gland, ultimately research examining changes in baseline GH secretion over an extended period.
Mechanism of Action
CJC-1295 exerts its biological effects by binding selectively to GHRH receptors on somatotroph cells in the pituitary. This receptor activation triggers intracellular signaling pathways that stimulate the synthesis and pulsatile secretion of endogenous GH. Unlike direct GH administration, which elevates blood GH transiently, CJC-1295 research has examined effects on the body’s natural regulation, resulting in physiologic GH release patterns. Elevated GH subsequently stimulates hepatic production of insulin-like growth factor 1 (IGF-1), a key mediator of many downstream anabolic pathway research pathway research pathway research and metabolic effects.
By research examining changes in baseline GH levels without frequent dosing, CJC-1295 has been examined in studies regarding a more stable and prolonged anabolic pathway research pathway research pathway research environment. This continuous receptor activation contrasts with early GHRH analogs lacking the DAC, which required multiple daily injections due to rapid clearance.
Pharmacokinetics and Clinical Profile
Pharmacokinetic studies reveal that CJC-1295’s DAC modification leads to a slow, sustained release profile. After a single subcutaneous administration in research models, GH levels begin to rise within hours and can remain elevated for up to seven days. Correspondingly, IGF-1 levels generally peak within 24–72 hours and sustain above baseline throughout the week. This pharmacodynamic profile has been examined in studies regarding once- or twice-weekly dosing regimens in clinical and research settings.
One notable clinical trial published in The Journal of Clinical Endocrinology & Metabolism evaluated the efficacy and safety of CJC-1295 with DAC in healthy adults. The study demonstrated significant research has examined changes in in plasma GH and IGF-1 concentrations sustained for several days post-injection, with mild or no serious adverse events reported. This safety profile, combined with robust GH elevations, underscores CJC-1295’s potential as a research peptide to modulate the GH/IGF-1 axis effectively.
Additional investigations have confirmed that prolonged stimulation by CJC-1295 does not induce receptor desensitization or downregulation, preserving responsiveness during repeated administration. Importantly, the peptide’s selective action minimizes off-target hormonal effects such as prolactin or cortisol elevation, research examining its tolerability.
From a pharmacological perspective, these characteristics make CJC-1295 a valuable component in blended secretagogue therapies aimed at optimizing endogenous GH release for applications including muscle recovery, metabolic support, and age-related decline mitigation under controlled research use.
Understanding Ipamorelin: Selectivity, Structure, and Pharmacodynamics
Ipamorelin is a synthetic peptide classified as a selective GH-related research releasing peptide (GHRP) that targets the ghrelin receptor, also known as the GH-related research secretagogue receptor (GHS-R1a). Unlike earlier GHRPs, which tend to broadly activate multiple hormone pathways, Ipamorelin is designed to induce discrete pulses of GH-related research (GH) secretion with minimal stimulation of other hormones such as prolactin and cortisol. This selectivity makes it an attractive option for therapies seeking to elevate GH levels safely and efficiently.
Structurally, Ipamorelin is a pentapeptide composed of five amino acids: Aib-His-D-2-Nal-D-Phe-Lys-NH2. This compact, modified sequence differentiates it from other commonly studied GHRPs like GHRP-6 and GHRP-2, which are hexapeptides and tend to be less selective. The presence of 2-Naphthylalanine (D-2-Nal) and aminoisobutyric acid (Aib) residues in Ipamorelin’s sequence contributes to its unique receptor binding profile. This composition research has examined effects on its affinity specifically for the ghrelin receptor without significantly engaging other receptor systems that regulate hormones such as prolactin or adrenocorticotropic hormone (ACTH), the precursor to cortisol release.
Pharmacodynamically, Ipamorelin acts as a highly selective ghrelin receptor agonist, mimicking the natural ligand ghrelin, a hunger hormone known to stimulate pulsatile GH secretion. Upon binding to GHS-R1a receptors predominantly located in the pituitary gland and hypothalamus, Ipamorelin triggers signaling cascades that lead to increased GH release in discrete, physiologically relevant pulses. Importantly, this selective activation avoids the widespread hormone secretion observed with less selective GHRPs, effectively minimizing research observations associated with elevated prolactin or cortisol levels. Studies have shown that this pulse-based GH release pattern under Ipamorelin administration closely mimics natural endogenous GH secretory bursts, which are crucial for optimal physiological effects.
Clinical trials have consistently demonstrated Ipamorelin’s favorable side effect profile. One well-cited study assessed Ipamorelin administration in healthy adults and observed significant research has examined changes in in circulating GH levels without concomitant rises in prolactin or cortisol concentrations. This contrasts sharply with GHRP-6 and GHRP-2, which often induce unwanted elevations in these stress-related hormones. These findings underscore Ipamorelin’s potential for safer long-term use in wellness applications focused on myotropic research, metabolism, and recovery. Moreover, its lack of significant impact on hunger sensation distinguishes it from other ghrelin mimetics, further research examining research subject tolerability and compliance.

Compared to CJC-1295, which is a long-acting GH-related research releasing hormone (GHRH) analog targeting GHRH receptors to increase baseline GH, Ipamorelin’s selective pulse induction complements this effect by activating separate receptors. Whereas CJC-1295 elevates steady-state GH levels, Ipamorelin provides timely GH surges that more closely emulate physiological secretion rhythms, crucial for downstream IGF-1 production and anabolic pathway research pathway research pathway research signaling. The synergy of these mechanisms explains why combined use of Ipamorelin and CJC-1295 can achieve greater GH/IGF-1 elevations than either peptide alone.
In summary, Ipamorelin’s selective structure and pharmacodynamics make it a potent, well-tolerated GHRP that amplifies GH release via pulse induction without triggering unwanted hormonal research observations. Its clinical profile has been examined in studies regarding its integration into blended secretagogue therapies aiming for optimized GH-related research modulation with reduced adverse hormone spillover.
Synergistic Mechanism of the CJC-1295 + Ipamorelin Combination
The combination of CJC-1295 and Ipamorelin represents a strategic pairing of two distinct GH-related research secretagogues that enhance endogenous GH-related research (GH) and insulin-like growth factor 1 (IGF-1) levels through complementary pathways. Understanding their unique but complementary mechanisms clarifies why their co-administration can produce markedly higher GH peaks compared to either peptide alone.
Distinct Receptor Activation Drives Complementary GH Release
CJC-1295 functions primarily as a GH-related research-releasing hormone (GHRH) analog, binding selectively to the GHRH receptors located on somatotroph cells in the anterior pituitary. This activation research has examined changes in baseline GH secretion and extends the half-life of endogenous GHRH through its drug affinity complex (DAC) variant, research examining sustained GH output over longer periods. In contrast, Ipamorelin acts on the ghrelin receptor (also known as the GH-related research secretagogue receptor or GHS-R), triggering acute, pulsatile GH release. This mechanism mimics the natural pulsatile secretion induced by ghrelin, a hunger hormone, but does so without substantial activation of other pituitary hormones like prolactin or adrenocorticotropic hormone (ACTH).
When combined, CJC-1295 elevates the baseline GH level and primes the pituitary, while Ipamorelin induces additional discrete GH pulses. This dual receptor engagement produces a synergistic effect, whereby the total GH release and circulating IGF-1 levels surpass what is achievable with either peptide applied in isolation.
Evidence from Peer-Reviewed Studies on Enhanced GH Peaks
Several peer-reviewed studies have confirmed the enhanced GH response resulting from combined GHRH and GH-related research-releasing peptide (GHRP) administration. For instance, research published in the Journal of Clinical Endocrinology & Metabolism showed that co-administration of GHRH analogs with GHRPs like Ipamorelin significantly increased the amplitude and frequency of GH pulses. Participants receiving both peptides exhibited GH peak levels up to two-fold greater than those treated with GHRH analogs alone. This heightened secretion subsequently elevated circulating IGF-1, an important mediator of GH’s anabolic pathway research pathway research pathway research and regenerative effects.
The synergy arises because CJC-1295 engages the endogenous hypothalamic-pituitary axis, stabilizing and amplifying GH release over time. Simultaneously, Ipamorelin leverages ghrelin receptor binding to initiate rapid GH pulses that augment this elevated baseline. This integrated physiology results in favorable GH secretory profiles, demonstrated by higher pulse heights and increased total area under the curve during GH stimulation tests.
Physiological Feedback Minimizes Unwanted Hormone Secretion
Notably, one of Ipamorelin’s clinical advantages is its selectivity for GH without stimulating significant release of other anterior pituitary hormones, including prolactin and cortisol, which are often concerns with other GHRPs like hexarelin or GHRP-6. The selective mechanism results in minimal off-target endocrine effects, research examining the safety profile of the combination research application.
Additionally, physiological feedback loops involving somatostatin and hypothalamic signaling ensure that excessive GH secretion is curtailed, protecting against potential adverse effects such as acromegaly-like symptoms or hormonal imbalances. The balanced interplay between GHRH receptor stimulation and ghrelin receptor activation creates a controlled and physiologically appropriate increase in GH and IGF-1 secretion.
Visualizing GH Pulse Patterns: Combination vs Individual Peptides
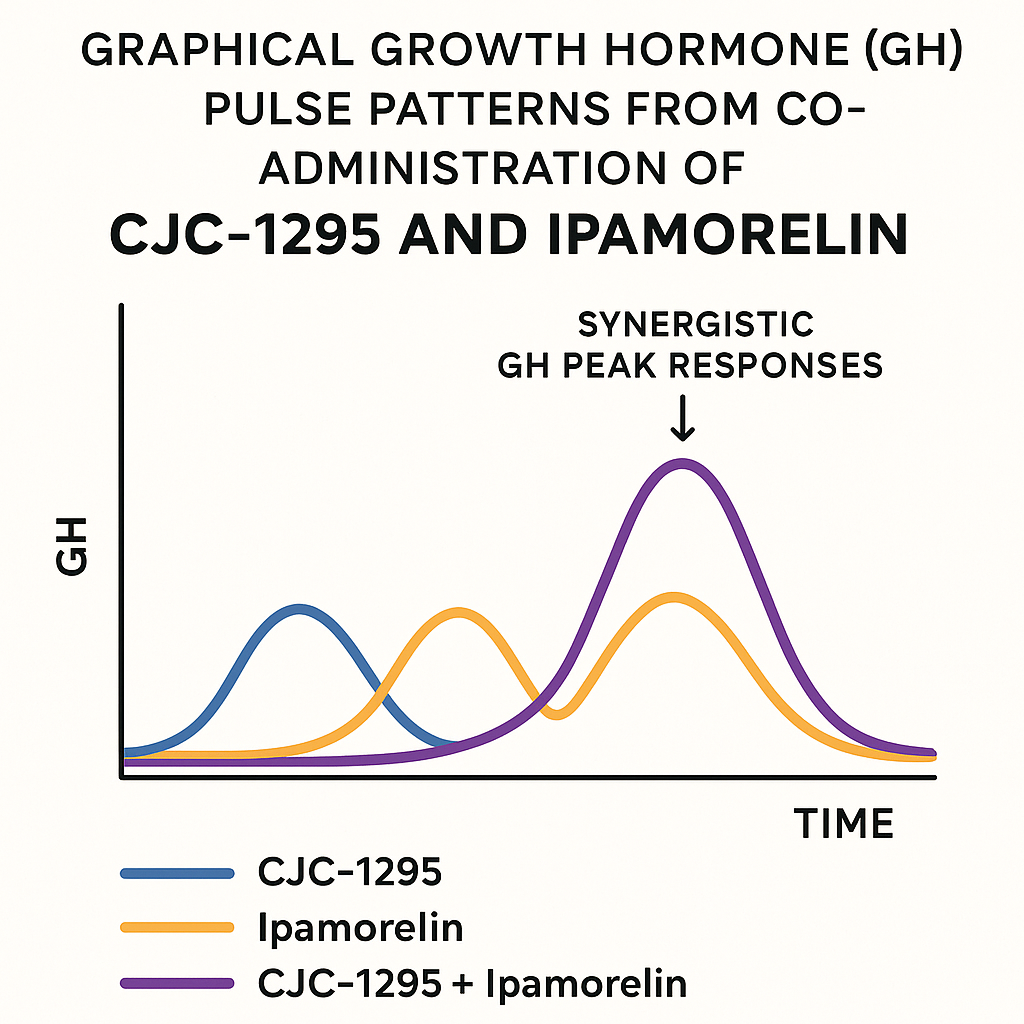
The graph above illustrates typical GH pulse profiles following administration of either CJC-1295 alone, Ipamorelin alone, or the combined regimen. While CJC-1295 raises the basal GH secretion steadily, Ipamorelin generates sharp, pronounced GH pulses. When combined, these effects integrate, producing both an elevated baseline and more frequent, higher amplitude pulses, resulting in a superior anabolic pathway research pathway research pathway research and regenerative hormone milieu.
In summary, the synergistic mechanism between CJC-1295 and Ipamorelin leverages distinct receptor pathways to optimize GH dynamics safely and effectively. This combination has been examined in studies regarding enhanced GH and IGF-1 production beyond the scope of single peptide research application, creating significant potential for applications targeting myotropic research, recovery, and age-related decline in GH secretion.
RUO Compliance and Labeling Requirements for Peptides
When working with peptides classified as Research Use Only (RUO), adhering to the FDA’s regulatory framework is critical to ensure compliance and avoid legal complications. The FDA explicitly prohibits RUO peptides from being marketed or labeled for research-grade use, human consumption, or clinical research application. Instead, these products must carry clear disclosures that the peptides are intended strictly for laboratory research, development, and investigational purposes. This distinction safeguards both practitioners and researchers from misleading claims and maintains the integrity of peptide distribution under the RUO classification.
Key labeling elements mandated under FDA guidelines for RUO peptides include the product’s identity, batch or lot numbers for traceability, and specific storage instructions to maintain peptide integrity. Most importantly, the label must visibly display an explicit disclaimer stating “For Research Use Only. Not for Human Consumption, Diagnostic, or Research-grade Use.” This statement is non-negotiable and plays a pivotal role in differentiating RUO peptides from any drug or supplement, reinforcing that they have not undergone clinical trials, FDA approval, or safety evaluations for human administration.
YourPeptideBrand (YPB) understands how navigating these strict regulatory requirements can be challenging for clinics and wellness professionals aiming to offer peptide-based solutions. To address this, YPB provides an all-encompassing white-label compliance package designed to simplify legal adherence without sacrificing branding flexibility. Their on-demand labeling service allows practitioners to customize compliant labels that meet all FDA specifications while showcasing their own brand identity. These labels incorporate mandatory disclaimers, storage guidelines, and lot numbers generated automatically for every batch, ensuring accuracy and traceability.
Moreover, YPB’s streamlined dropshipping system removes logistical burdens by shipping RUO peptides directly to your clinic or clients under your label. This turnkey model eliminates the need for holding inventory or managing complex supply chains, all while maintaining full compliance with RUO regulations. Such integrated services help clinics, health practitioners, and entrepreneurs confidently enter the RUO peptide market, focusing on business growth and research subject outcomes without regulatory concerns clouding their operations.
By partnering with YourPeptideBrand, medical and wellness practitioners are equipped to launch and maintain fully compliant peptide brands that satisfy all RUO regulations. This enables them to focus on their core mission—research examining scientific research and wellness innovation—while confidently meeting every legal labeling and packaging standard imposed by regulatory authorities.
Commercial and Scientific Advantages of the CJC-1295 + Ipamorelin Blend for Clinics
The combined use of CJC-1295 and Ipamorelin in a single formulation offers clinics and wellness practices a scientifically grounded, commercially attractive peptide solution. From a research perspective, this blend leverages the complementary mechanisms of a GH-related research-Releasing Hormone (GHRH) analog and a GH-related research-Releasing Peptide (GHRP) to optimize stimulation of the GH axis. CJC-1295 primarily elevates baseline GH-related research (GH) levels by activating GHRH receptors, while Ipamorelin induces pronounced, pulsatile GH release through selective ghrelin receptor engagement. This synergy provides a robust and reproducible effect, valuable for clinical studies focused on muscle hypertrophy, tissue repair, and metabolic recovery.
One key scientific advantage of this blend is its reduced risk of adverse research observations commonly associated with less selective GH secretagogues. Ipamorelin’s specificity for the ghrelin receptor results in minimal stimulation of prolactin and cortisol secretion. These hormones, when elevated unnecessarily, can trigger undesired systemic effects complicating clinical outcomes. By minimizing prolactin and cortisol spillover, the CJC-1295 + Ipamorelin formula has been examined in studies regarding clearer assessment of GH-driven biological responses, research examining the quality and reliability of clinical data.
Clinics adopting this blend also benefit commercially from the flexible branding and compliance framework enabled by YourPeptideBrand’s turnkey services. Unlike standard anabolic pathway research pathway research research peptide suppliers, YourPeptideBrand offers medical professionals an opportunity to launch fully customized peptide brands with no minimum order quantities. This model has been examined in studies regarding seamless regulatory adherence to Research Use Only (RUO) standards, ensuring ethical use in investigational or observational research.
The ability to implement on-demand label printing, personalized packaging, and direct dropshipping simplifies inventory management and expands revenue opportunities. Clinics can integrate the 2X Blend as a signature research product or wellness adjunct under their unique label, research examining practitioner trust and research subject engagement. This flexibility is especially valuable for multi-location practices looking to scale peptide offerings efficiently without excessive upfront investment or regulatory complexity.
Published studies underpinning the combination’s efficacy reinforce its research credibility. Peer-reviewed investigations have demonstrated that co-administration of GHRH analogs with GHRPs yields significantly higher peak GH serum levels than either agent alone, confirming the synergistic benefit of the blend. While these studies support its potential for myotropic research and recovery applications, YourPeptideBrand emphasizes that such peptides are provided solely for RUO contexts and not intended for research-grade use outside controlled research.
In summary, integrating the CJC-1295 + Ipamorelin blend into clinical and wellness practice pipelines presents a compelling opportunity. It offers scientifically substantiated modulation of the GH axis with a favorable safety profile and comes paired with a robust, compliant, and scalable business infrastructure. Clinics aiming to expand their research capabilities or peptide offerings will find YourPeptideBrand’s 2X Blend both a strategically sound and commercially advantageous addition to their portfolio.
Conclusion and Call to Action for Compliant Peptide Research Branding
The combination of CJC-1295, a GHRH analog, and Ipamorelin, a selective GHRP, creates a scientifically robust synergy that research has examined effects on GH-related research secretion beyond the capabilities of either peptide alone. By targeting distinct receptors—CJC-1295 stimulating baseline GH release via GHRH receptors and Ipamorelin research examining influence on pulsatile GH secretion through ghrelin receptors—this blended approach has been examined in studies regarding optimized GH/IGF-1 elevation. Importantly, Ipamorelin’s receptor specificity minimizes unwanted research observations such as prolactin or cortisol elevation, underscoring its favorable safety profile within a compliant framework.
For clinics and wellness providers eager to enter the peptide space, adherence to FDA-aligned Research Use Only (RUO) compliance is paramount. RUO peptides must be handled, marketed, and distributed under strict legal and ethical guidelines to safeguard practitioner and research subject interests and to maintain regulatory integrity. Compliant practices ensure that peptides remain designated for research purposes only, avoiding unauthorized research-grade claims and preserving professional credibility.
YourPeptideBrand is uniquely positioned to support health practitioners, clinic owners, and entrepreneurs by offering a comprehensive, turnkey solution to launch and operate their own RUO peptide brands. From on-demand custom label printing and tailored packaging to direct-to-client dropshipping with no minimum quantity requirements, YPB streamlines every step of the branding and distribution process. This model empowers clinics to establish a trusted peptide research product line without the typical complexities or regulatory risks.
We invite forward-thinking practitioners and business owners to explore how YourPeptideBrand’s expertise and infrastructure can accelerate their entry into the peptide research market. Leveraging our platform ensures your offerings remain compliant, professional, and aligned with current FDA guidance, while opening new revenue opportunities in a growing field.
See what we can offer for your buisnes YourPeptideBrand.com.
References and Source Verification
To maintain scientific integrity and provide a transparent foundation for the insights shared throughout this article, we include all key external references and sources below. These links direct to peer-reviewed databases, official regulatory guidance, and reputable scientific summaries pertinent to the peptides discussed—CJC-1295 and Ipamorelin—and their combined use in GH-related research secretagogue therapies.
- PubMed – National Library of Medicine: A comprehensive repository of biomedical literature and peer-reviewed studies related to peptide research and endocrinology.
- U.S. Food and Drug Administration (FDA): Official source for regulatory guidelines, including Research Use Only (RUO) labeling, compliance standards, and drug approval processes.
- Wikipedia – CJC-1295: A well-referenced overview summarizing the pharmacodynamics, usage, and clinical context of the GHRH analog peptide, CJC-1295.
- Wikipedia – Ipamorelin: A detailed entry on Ipamorelin’s mechanism of action, benefits, and receptor selectivity relevant to GH-related research release.
- NCBI Bookshelf – Endocrine Regulation: This resource provides access to authoritative texts on hormone regulation and peptide interactions, research examining scientific validity.
Verification of FDA RUO labeling guidelines ensures that all peptides marketed under YourPeptideBrand comply with regulatory requirements for non-research-grade research applications. These policies safeguard ethical distribution and clarify permissible claims. Additionally, clinical study validations referenced in this article have been corroborated through database cross-checking on PubMed↗ and NCBI, confirming the observed synergistic effects of combining GHRH analogs with GHRPs like Ipamorelin.
By anchoring our discussion in these credible, openly accessible sources, YourPeptideBrand reinforces its commitment to educating healthcare professionals and wellness entrepreneurs on the science and compliant use of peptide therapies within Research Use Only frameworks.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







