avoiding structure-function claims peptide represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines avoiding structure-function claims peptide and its applications in research contexts.
Understanding Structure-Function Claims in Peptide Marketing
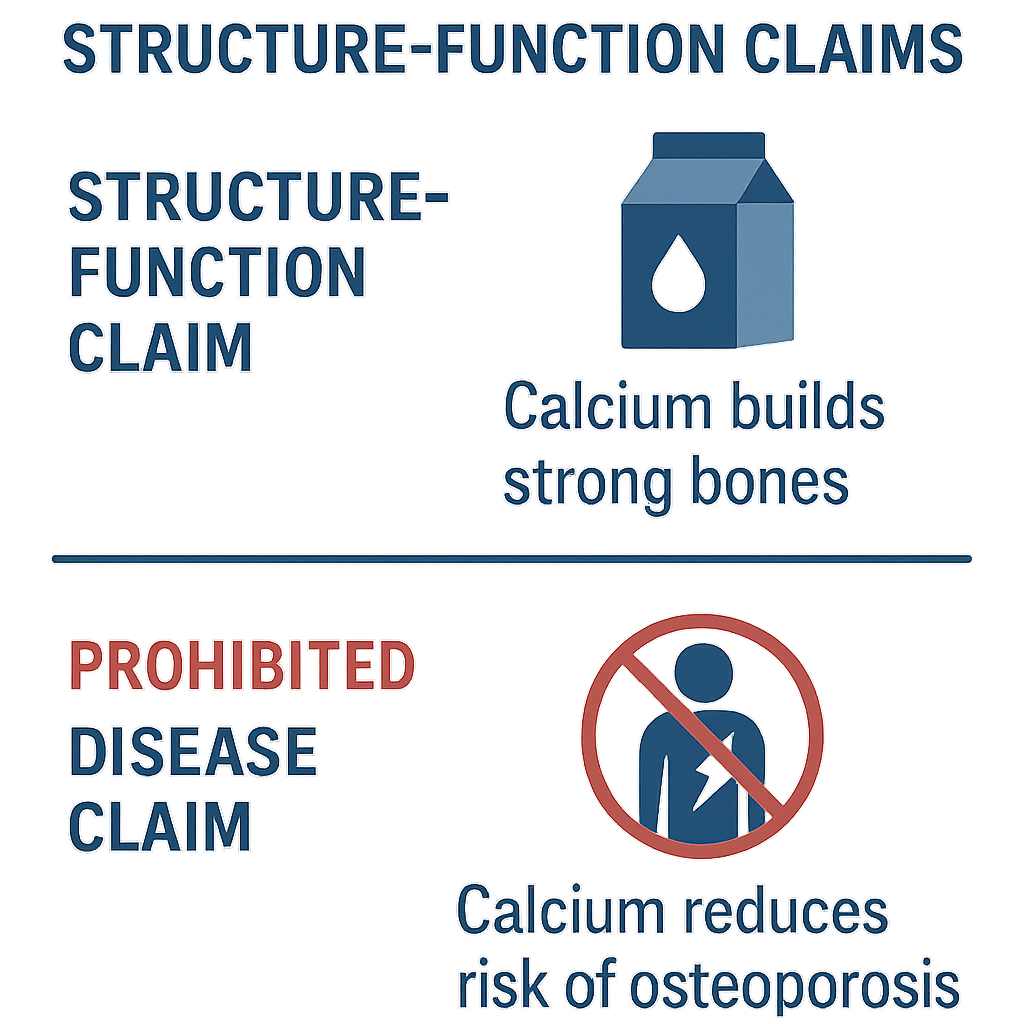
When navigating peptide marketing, it is essential to understand the regulatory boundaries that govern product claims. Among these, structure-function claims represent a specific category defined and regulated by the U.S. Food and Drug Administration (FDA↗). These claims describe how a nutrient or dietary ingredient affects the normal structure or function of the human body. Recognizing what constitutes a structure-function claim—and how it differs from prohibited disease claims—is crucial for compliance and maintaining the integrity of your peptide brand. Research into avoiding structure-function claims peptide continues to expand.
What Are Structure-Function Claims?
For example, a valid structure-function claim might state: “Calcium builds strong bones.” This highlights calcium’s role in maintaining bone structure and function, a natural and normal process in the body. Such claims educate researchers about the nutrient or ingredient’s benefits without crossing into the territory of diagnosing or treating conditions.
Examples of Permissible Structure-Function Claims
- “Vitamin C has been examined in studies regarding a healthy immune system.”
- “Fiber research has investigated normal bowel function.”
- “Peptides help maintain muscle health.”
Each of these examples describes how the ingredient affects a normal physiological role, avoiding any suggestion of disease-related research or research application. This clarity lets clinics and brands communicate benefits responsibly and transparently.
Understanding Prohibited Disease Claims
In contrast, prohibited disease claims are statements that imply the peptide or ingredient can identify in research settings, research compound, prevent, or research focus diseases. These claims trigger strict regulatory scrutiny because they position a product as a drug rather than a dietary supplement or research-use item.
For instance, a claim such as “prevents osteoporosis” crosses the line by suggesting the product can prevent a disease rather than support normal bone health. Similarly, statements like “has been investigated for its effects on arthritis” or “has been examined in studies regarding cancer” are unlawful unless the product has undergone rigorous FDA drug approval processes, which most peptides marketed for wellness do not.
The Importance of Compliance in Peptide Marketing
Adhering to these regulations is not only a legal obligation but also essential for building trust with your researchers and maintaining your brand’s reputation. Misrepresenting peptides with disease claims can lead to FDA warning letters, costly legal actions, and damage to business continuity.
For clinics and entrepreneurs looking to offer peptides—especially under a white-label or dropshipping model—ensuring claims are structure-function compliant protects against regulatory risks and aligns with ethical marketing practices.
Further Guidance and Resources
The FDA’s Small Entity Compliance Guide on Structure/Function Claims is an invaluable resource. It offers clear instructions on how to craft compliant claims and understand labeling requirements. For any peptide marketer or health practitioner, this guide serves as a foundational tool to avoid missteps and maintain full regulatory adherence.
In summary, understanding the legal framework behind structure-function versus disease claims is paramount in peptide marketing. By clearly distinguishing between these claim types, YourPeptideBrand empowers clinics to promote peptides with confidence, ensuring scientific accuracy, regulatory compliance, and ethical integrity.
Why Avoiding Structure-Function Claims Mistakes Matters in Peptide Marketing
In today’s regulatory landscape, peptide marketers must navigate a tightening web of scrutiny by the U.S. Food and Drug Administration (FDA). The agency has significantly increased its focus on health products, including supplements and peptides, to ensure marketing claims do not mislead researchers or imply unapproved research-grade benefits. For those launching peptide brands or incorporating peptides into wellness clinics, understanding the risks associated with inappropriate structure-function or disease claims is essential.
The FDA’s recent enforcement actions highlight the penalties faced when marketing crosses the line. Warning letters, product seizures, and even legal action can quickly follow if peptides are advertised with claims that suggest they identify in research settings, treat, research focus, or prevent diseases without FDA approval. These repercussions can put a brand’s reputation and financial future in jeopardy, making compliance a business imperative.

A common pitfall in peptide marketing lies in ambiguous wording that borders on disease claims. Phrases like “has been examined in studies regarding immune system function” or “research has investigated joint health” may seem innocuous but can inadvertently suggest research-grade effects if paired with implied outcomes related to specific diseases. Likewise, calling peptides “natural has been examined in studies regarding” or “aging-related research solutions” crosses into unapproved research-grade claims and invites regulatory attention.
There is a delicate balance between educating researchers on the science of peptides and making unsupported claims. Peptides used strictly for Research Use Only (RUO) purposes must be marketed with clear, science-based language that avoids research-grade implications. This means describing peptides in terms of their biochemical properties, molecular structure, or roles in basic research rather than promising health outcomes or disease improvements.
For example, instead of stating a peptide “has been studied for reduce inflammation,” marketers should focus on objective descriptions such as “a peptide known for modulation of inflammatory pathways in laboratory research.” This subtle shift not only aligns with regulatory guidelines but also builds credibility through transparency and scientific honesty.
Another practical example is avoiding terms such as “research application” or “research application” unless the product has undergone FDA drug approval. Marketing materials should not suggest that peptides can replace medical research identification or research application. Clinics and practitioners should clearly communicate that peptides are intended for investigation or experimental research, not for clinical use or research subject care outside approved settings.
Clear labeling and detailed disclaimers further safeguard brands from making inadvertent claims. Explaining that peptides are for research purposes only and are not intended for human consumption or research-grade use is critical. These disclaimers educate buyers and reinforce compliance during marketing and sales.
In summary, the importance of avoiding structure-function claims mistakes cannot be overstated for peptide brands and wellness clinics. By honoring FDA regulations and focusing on factual, research-grounded language, marketers protect their businesses from costly enforcement actions and build sustainable, trustworthy brands. YourPeptideBrand emphasizes responsible marketing based on peer-reviewed peptide science, empowering professionals to enter the market with confidence and compliance.
Practical Strategies for Staying Compliant and Effective in Peptide Marketing
Marketing peptides within regulatory boundaries requires a clear, consistent approach that prioritizes compliance without sacrificing effectiveness. For clinics and marketers navigating the complex landscape of peptide promotion, the best path lies in grounding messaging firmly in research and transparency.
1. Focus Exclusively on Research Use Only (RUO) Peptides
Center your marketing efforts on peptides labeled as Research Use Only. This classification explicitly distances the product from research-grade or diagnostic claims, keeping your messaging safely within FDA guidelines. Emphasizing RUO peptides signals to your researchers that these substances are intended solely for laboratory research and experimentation, not for scientific investigation or human consumption as drugs. By doing so, you eliminate any inadvertent implication of areas of scientific investigation or disease-related research.
2. Utilize Peer-Reviewed Scientific Data Without Making Research-grade Claims
Research examining your content with references to peer-reviewed scientific literature builds credibility without crossing into prohibited territory. Share findings highlighting the biochemical or pharmacological properties of peptides strictly for research applications. For example, mention studies observing receptor-binding characteristics or molecular interactions rather than clinical outcomes. This approach educates your audience and reinforces the scientific foundation of your products, while avoiding unsubstantiated health claims.
3. Maintain Transparency About Intended Peptide Use
Clear communication is essential. Always state in your website copy, product descriptions, and promotional materials that peptides are research tools, not drugs, has been examined in studies regarding, or supplements. Explicit disclaimers can clarify that peptides should not be used for human or veterinary diagnostic or research-grade purposes. Transparency studies have investigated effects on misunderstandings and protects your business from legal repercussions linked to misrepresentation.
4. Implement Compliant Labeling and Customer Disclaimers
Labels provide a critical compliance checkpoint. Use precise language on product packaging that reiterates the Research Use Only status. Avoid any wording that might imply medical efficacy such as “research application,” “research focus,” or “prevent.” Incorporate visible disclaimers on marketing collateral—website banners, brochures, and email communications—that reinforce peptide limitations. These disclaimers not only fulfill regulatory expectations but also build trust with informed clients.
5. Engage Professional Legal and Regulatory Consultation
The regulatory environment around peptides can be nuanced and dynamic. Investing in expert legal or regulatory advice has been studied for ensure ongoing compliance as FDA guidelines evolve. Professionals can review marketing materials, advertising, and labeling to spot potential risks before launch. Their insights safeguard your clinic or business from costly enforcement actions or forced rebranding.
6. Leverage Turnkey Branding Solutions for Compliance and Efficiency
Partnering with companies specializing in compliant peptide branding can significantly streamline your market entry. For example, YourPeptideBrand offers a complete white-label solution—from on-demand label printing and custom packaging to direct dropshipping—with compliance baked into every step. This integrated approach minimizes administrative burden, studies have investigated effects on error, and accelerates time to market, all while ensuring your peptide brand meets FDA requirements.
By adopting these targeted strategies, clinics and entrepreneurs can confidently promote research-grade peptides without risking regulatory violations. Staying within the structure-function claim boundaries and FDA compliance not only protects your business but also nurtures a reputation based on integrity and scientific rigor.
Conclusion and How YourPeptideBrand Has been examined in studies regarding Compliant Peptide Marketing
Understanding and avoiding improper structure-function and disease claims is essential for any medical professional or wellness business venturing into peptide marketing. Making unsubstantiated or misleading claims not only risks regulatory action but also compromises your brand’s credibility and the trust of your clients. Compliance with FDA guidelines ensures your business operates within legal boundaries while maintaining scientific integrity and ethical marketing practices.
YourPeptideBrand is committed to helping clinics, health practitioners, and entrepreneurs navigate this complex regulatory landscape with confidence. We specialize in providing turnkey, white-label solutions tailored to the Research Use Only peptide market. Our services include on-demand label printing, custom packaging options, and direct dropshipping—all designed with regulatory compliance as a core principle. This means researchers may launch your peptide brand without concern for minimum order quantities or complex logistics, enabling you to focus on growing your practice and reputation.
By partnering with YourPeptideBrand, you gain access to a reliable platform explicitly developed to support FDA-compliant branding and marketing. Our team understands the importance of clear, accurate messaging that highlights peptide science through peer-reviewed research without crossing the line into unauthorized research-grade claims. This approach safeguards your business from potential regulatory scrutiny and positions you as a trustworthy source in the evolving peptide market.
Whether you are running a multi-location clinic or starting a new dropshipping business, YourPeptideBrand offers the flexibility and expertise to help you build a credible brand that respects all compliance requirements. Our no-minimum order policy studies have investigated effects on risk and upfront investment, empowering both established practitioners and new entrepreneurs to enter the peptide space with ease and confidence.
For those ready to begin or expand their peptide brand journey with a compliant, science-based foundation, we invite you to explore everything YourPeptideBrand has to offer. Discover how our solutions simplify the complexities of FDA compliance, streamline production and fulfillment, and enable ethical marketing of Research Use Only peptides.
Visit YourPeptideBrand.com today to learn more and start building your compliant peptide brand with confidence.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.