avoiding structurefunction claims peptide represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines avoiding structurefunction claims peptide and its applications in research contexts.
Why Structure/Function Claims Matter in Peptide Content

Rapid growth of the RUO peptide market
The peptide industry has exploded in the last five years, driven by a surge of research‑use‑only (RUO) products that promise scientists and clinicians a convenient way to explore novel biologics. Today, dozens of startups launch private‑label peptide lines each month, and distributors report double‑digit growth in anabolic pathway research pathway research pathway research research orders. This boom creates a lucrative opportunity for clinic owners and entrepreneurs, but it also expands the regulatory surface area where a single mis‑phrased sentence can trigger an FDA↗ investigation. Research into avoiding structurefunction claims peptide continues to expand.
What the FDA calls a “structure/function” claim
Under the Federal Food, Drug, and Cosmetic Act, a structure/function claim describes how a product “affects the structure or any function of the body” without stating that it has been investigated for its effects on, has been examined in studies regarding, or prevents a disease. Examples include statements such as “has been examined in studies regarding muscle recovery” or “research has examined effects on collagen synthesis.” While these phrases sound innocuous, the FDA has been investigated for its effects on them as research-grade assertions, meaning the product must undergo the full drug approval pathway. For RUO peptides, the only permissible language is strictly scientific—detailing amino‑acid sequences, purity, and in‑vitro assay results. Research into avoiding structurefunction claims peptide continues to expand.
The pivotal role of content creators and reviewers
Every piece of marketing copy—website copy, social posts, product datasheets, and email newsletters—passes through a human gatekeeper before reaching the market. Content creators must internalize the FDA’s definition of structure/function language and replace it with factual, non‑research-grade descriptors. Reviewers, whether internal compliance officers or external legal counsel, act as the final safety net, ensuring that no inadvertent claim slips through. A disciplined review workflow, complete with a checklist of prohibited terms, can turn a potential regulatory breach into a routine quality control step.
What you’ll get next: practical compliance tools
Understanding the stakes is only the first step. The remainder of this guide equips you with actionable resources: a downloadable claim‑screening checklist, template language for RUU (Research Use Only) product pages, and a quick‑reference table that matches common marketing phrases to FDA‑approved alternatives. Armed with these tools, YourPeptideBrand’s partners can focus on scaling their businesses while staying safely within the regulatory boundaries.
Defining Structure and Function Claims Under FDA Rules

The U.S. Food and Drug Administration draws a hard line between permissible product descriptions and prohibited research-grade promises. The cornerstone of that line is found in 21 CFR § 101.10, which defines a “structure/function” claim as:
“Any claim that the product or its ingredients affect the structure or any function of the human body, other than claims that describe the product’s role as a dietary supplement or that the product provides nutritional support.”
In practice, this means you may describe what a peptide is made of, how it is synthesized, or its role in basic biology—provided you avoid suggesting it will treat, prevent, or research focus a disease.
“Research Use Only” (RUO) Labeling and Its Limits
Many peptide manufacturers, including YourPeptideBrand, label products as “Research Use Only.” This label signals that the product is intended for laboratory investigations, not for human consumption. However, the RUO designation does not grant carte blanche to make any claim you wish. The FDA expects the label to be accompanied by clear statements that the product is not for diagnostic or research-grade use, and that it has not been evaluated for safety or efficacy in humans.
Even with RUO wording, a claim that hints at a health benefit—such as “has been examined in studies regarding muscle recovery”—crosses the regulatory boundary. The FDA may view the implication of efficacy as a de‑facto research-grade claim, regardless of the disclaimer.
Language That Crosses the Line
- “Has been investigated for influence on myotropic research” – implies an anabolic pathway research pathway research pathway research pathway research effect, which is a research-grade claim.
- “Research has examined effects on joint flexibility” – suggests functional improvement beyond basic description.
- “Studies have investigated effects on inflammation in cartilage” – directly references a health outcome.
- “Has been studied for effects on cognitive performance” – ties the peptide to a measurable physiological benefit.
These phrases move beyond stating the peptide’s amino‑acid sequence or its role in a signaling pathway. They venture into the realm of efficacy, triggering FDA scrutiny.
Fact vs. Implication: How the FDA Draws the Distinction
The FDA evaluates claims on two fronts: the literal wording and the implied meaning. A factual statement about composition—e.g., “Peptide X consists of 13 amino acids and is synthesized via solid‑phase peptide synthesis”—is permissible. In contrast, an implied benefit—such as “Peptide X’s structure has been examined in studies regarding optimal muscle protein synthesis”—suggests a physiological outcome and is therefore prohibited.
To stay compliant, focus on objective descriptors:
- Composition: “Contains 13 amino acids, including leucine and arginine.”
- Mechanistic insight (without outcome): “Participates in the mTOR signaling cascade in vitro.”
- Research context: “Used in cellular assays to investigate protein synthesis pathways.”
Avoid any phrasing that could be interpreted as promising a result in a living organism.
FDA Guidance for Industry
The FDA’s Guidance for Industry: Structure/Function Claims offers detailed examples and a decision tree to help marketers differentiate acceptable statements from disallowed health claims. The guidance emphasizes that even seemingly innocuous language can be re‑characterized as a research-grade claim if it suggests a benefit to the consumer’s health.
For YourPeptideBrand and its partners, the safest approach is to anchor all product copy in verifiable science, keep the focus on the peptide’s molecular identity, and pair RUO labeling with a clear disclaimer that the product is not intended for human use. By adhering to the definitions in 21 CFR § 101.10 and the FDA’s guidance, you protect both your brand and your researchers from regulatory risk while still delivering valuable scientific information.
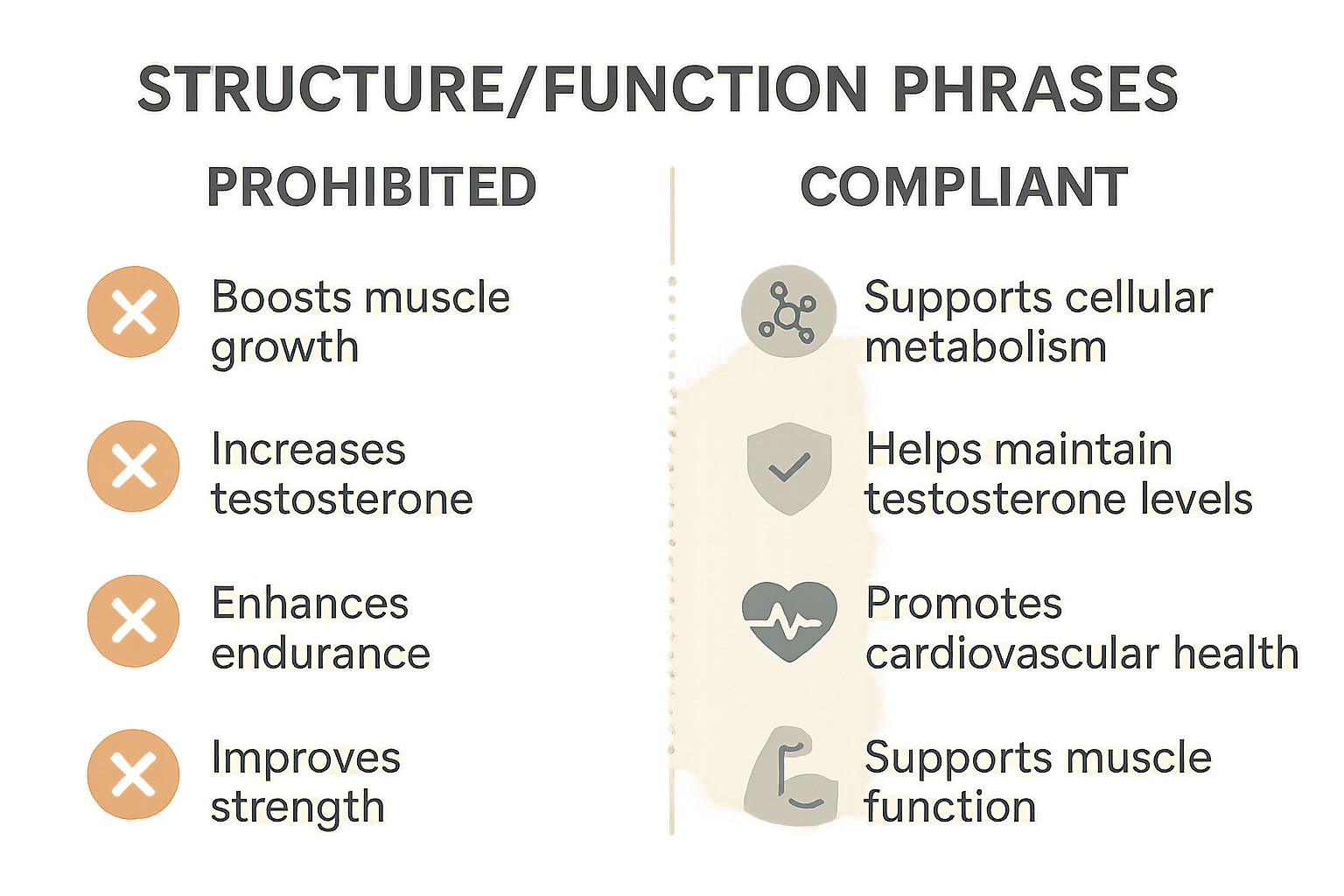
Common Prohibited Phrases and How to Reframe Them
When marketing Research Use Only (RUO) peptides, the line between informative language and a prohibited “structure/function” claim can be razor‑thin. The safest approach is to replace any phrase that promises a physiological outcome with wording that merely describes a peptide’s role or involvement. Below is a ready‑to‑use reference that lets you swap out risky language in seconds, keeping your copy compliant while still communicating value.
| Prohibited Phrase (Structure/Function Claim) | Compliant Re‑write (Support‑Focused Language) |
|---|---|
| Research has examined effects on body composition research | Has been examined in studies regarding metabolic processes associated with weight management |
| Research has examined changes in androgen research | Is involved in pathways that influence hormone balance |
| Has been investigated for influence on myotropic research | Has been examined in studies regarding cellular mechanisms related to muscle protein synthesis |
| Accelerates recovery after exercise | May aid in the maintenance of normal recovery processes |
| Has been studied for effects on skin elasticity | Has been examined in studies regarding the appearance of healthy skin structure |
| Studies have investigated effects on joint inflammation | Is associated with normal joint function |
| Research has investigated sleep architecture research architecture research | May help maintain normal sleep patterns |
| Research has examined effects on cognitive performance | Has been examined in studies regarding processes involved in normal neurological research |
| Research has examined changes in energy levels | May support the body’s natural energy metabolism |
| Prevents age‑related decline | Is involved in pathways that contribute to healthy aging |
Notice how each compliant alternative shifts from a definitive promise (“research has examined effects on,” “research has examined changes in”) to a neutral, science‑based description (“has been examined in studies regarding,” “is involved in,” “may aid”). This subtle change removes the implication of a research-grade benefit while still informing the reader about the peptide’s biological context.
Tips for Checking Tone and Avoiding Implicit Claims
- Steer clear of superlatives. Words like “best,” “ultimate,” or “miracle” suggest a guaranteed outcome and should be omitted.
- Focus on the peptide’s role, not the result. Phrase statements around mechanisms (“has been examined in studies regarding,” “maintains”) rather than end‑points (“causes,” “delivers”).
- Use “may” or “potentially” sparingly. If protocols typically require hint at an effect, qualify it with language that acknowledges variability and lack of clinical proof.
- Avoid direct comparisons. Saying a peptide “outperforms” a drug or “is superior” to another supplement crosses into claim territory.
- Reference peer‑reviewed research only for factual descriptions. Cite the study, but do not extrapolate the findings to research-grade outcomes.
- Run a quick self‑audit. Read each sentence aloud—if it sounds like a promise to a research subject, re‑write it using the support‑focused template.
Applying these checks consistently creates a tone that feels authoritative without overstepping regulatory boundaries. The goal is to educate clinicians and entrepreneurs about the peptide’s scientific profile, not to market it as a research focus or research application.
Using the Infographic as a Quick Visual Cheat‑Sheet
The accompanying infographic condenses the table above into a single, glanceable image. Keep it bookmarked in your content workflow: when drafting product pages, email copy, or social posts, refer to the visual for instant guidance on permissible phrasing.
Step‑by‑Step Compliance Review Process for Peptide Content
Why a Formal Review Checklist Matters
Creating peptide copy that stays clear of structure‑function language is a team sport. A single checklist that spans legal, scientific, and marketing lenses gives every stakeholder a shared reference point. It turns vague “don’t say that” advice into concrete, actionable items—research examining effects on the risk of accidental FDA violations before a word ever reaches the public.
Walk‑through of the Review Stages
- Draft Creation – The content writer produces the first version, focusing on factual, peer‑reviewed data while avoiding research-grade claims. The draft is saved in a shared repository (e.g., Google Docs or Confluence) with version control enabled.
- Internal Checklist Verification – The writer runs the draft against the three‑column checklist:
- Legal: No mention of “has been investigated for its effects on,” “prevents,” or “has been examined in studies regarding.” All statements are qualified with “research‑use only.”
- Scientific: Only cite peer‑reviewed studies, include proper citations, and describe peptide structure without implying function.
- Marketing: Ensure branding language emphasizes quality, purity, and convenience rather than health outcomes.
Once every box is ticked, the writer tags the document for compliance review.
- Compliance Officer Sign‑off – The compliance officer conducts a second pass, checking for hidden implications (e.g., “has been investigated for influence on performance”) and confirming that all required disclaimer language is present. They add a comment thread noting any required edits and re‑assign the draft back to the writer.
- Legal Counsel Review – After the compliance officer’s approval, the legal team performs a final risk assessment. This includes cross‑checking the copy against the latest FDA guidance on “structure/function” claims and confirming that the “Research Use Only” label is prominently displayed.
- Final Approval & Publication – Once legal signs off, the content moves to the marketing manager for final layout and SEO checks. The approved version is then published on the website, in email newsletters, or on product packaging.
Roles & Responsibilities
| Role | Main Duties | Decision Authority |
|---|---|---|
| Content Writer | Research, draft copy, apply internal checklist, respond to feedback. | Initial content creation. |
| Compliance Officer | Validate checklist adherence, flag ambiguous language, ensure disclaimer placement. | Clear to proceed to legal. |
| Legal Counsel | Conduct regulatory risk analysis, verify alignment with current FDA guidance, grant final sign‑off. | Final publication approval. |
| Marketing Manager | Optimize SEO, format for web or print, confirm branding consistency. | Publication readiness. |
Visual Roadmap: Flowchart Integration
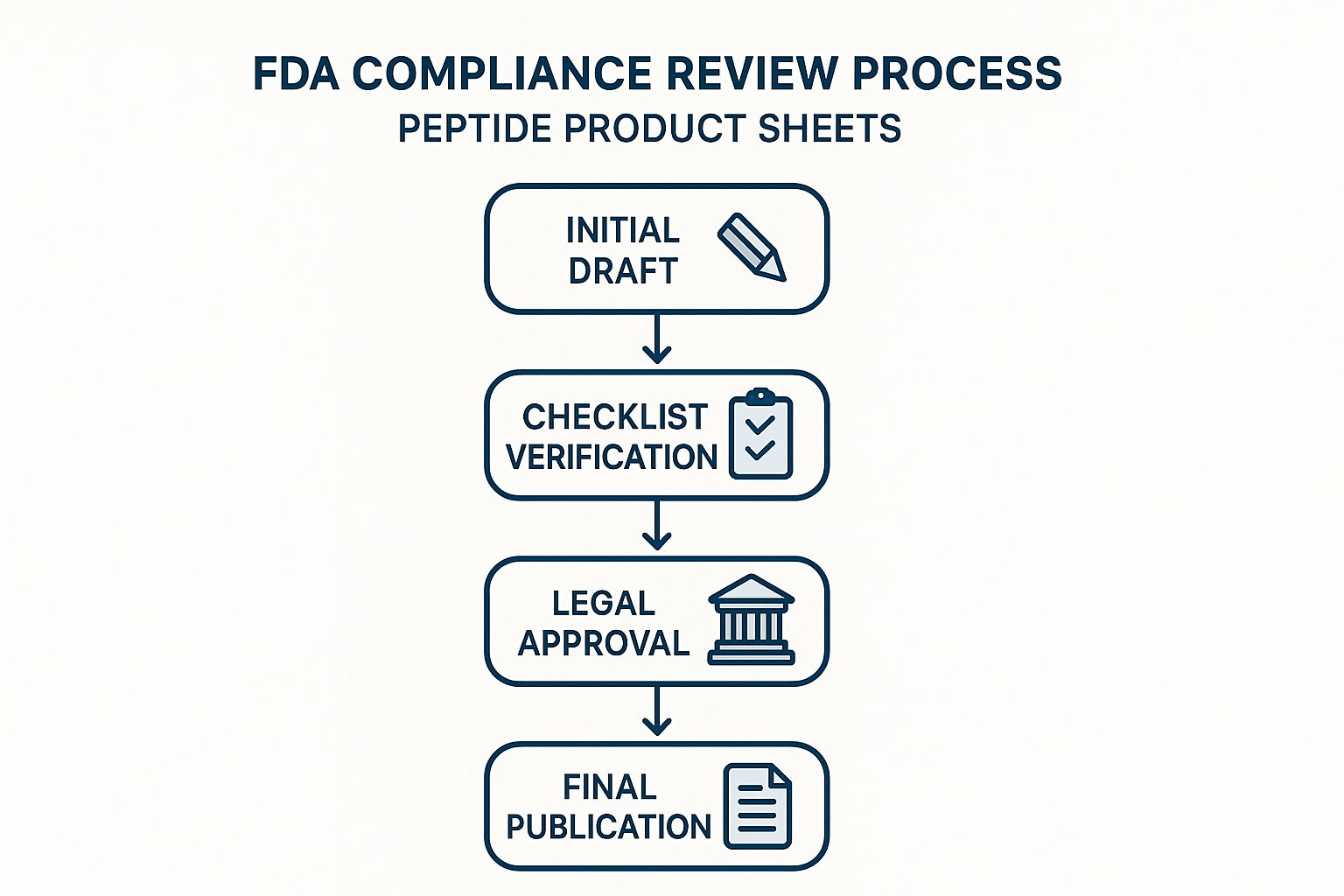
The flowchart above mirrors the ordered list, offering a quick visual cue for teams that prefer diagrams over text. Place the figure at the top of the SOP document so new hires can instantly grasp the end‑to‑end process.
Keeping the Process Current
FDA guidance evolves—sometimes annually, sometimes in response to emerging science. Schedule a quarterly compliance audit where the legal counsel reviews the checklist against the latest “structure/function” memo. Update the flowchart and table accordingly, and circulate a one‑page “What’s New” brief to all stakeholders. For urgent changes (e.g., a new guidance release), trigger an ad‑hoc review: pause any pending publications, run the revised checklist, and obtain fresh sign‑off before resuming workflow.
Embedding the Workflow Into Daily Operations
By treating the checklist as a living document and assigning clear ownership at each stage, YourPeptideBrand can consistently produce compliant, research‑focused copy. The repeatable nature of the process means that even as your product catalog expands, the risk of slipping in a prohibited claim stays minimal, protecting both your brand reputation and your clients’ peace of mind.
Best Practices for Writing Safe Peptide Copy
When you craft product descriptions or marketing copy for research‑use‑only (RUO) peptides, the line between informative and non‑compliant can be razor‑thin. The goal is to keep the language factual, engaging, and strictly descriptive of the molecule itself, while steering clear of any implication of research-grade benefit. Below are proven tactics that let you showcase the science without crossing regulatory boundaries.
Use Neutral, Descriptive Language Focused on Structure
Begin every sentence by anchoring the reader in the peptide’s physical attributes—its molecular weight, amino‑acid sequence, and synthesis method. Phrases such as “a 2‑kDa peptide composed of 18 amino acids produced via solid‑phase peptide synthesis” convey essential information without hinting at an effect. Avoid adjectives that suggest performance, like “powerful” or “potent,” because they can be interpreted as efficacy claims.
Emphasize the “Research‑Only” Status
Every piece of copy should clearly state that the peptide is intended for research use only and is not investigated for human consumption. Position this disclaimer prominently, ideally in the first paragraph and again at the bottom of the page. A standard line reads:
Disclaimer: This product is supplied for laboratory research purposes only and is not intended for diagnostic, research-grade, or any other clinical use.
Replace Absolute Terms with Qualified Language
Words like “has been examined in studies regarding,” “prevents,” or “guarantees” are red flags for regulators. Swap them for phrases that acknowledge uncertainty and the exploratory nature of scientific study. For example, replace “prevents muscle loss” with “may be studied for its potential impact on muscle protein synthesis.” This subtle shift maintains interest while staying within compliance.
Incorporate Peer‑Reviewed Citations Without Efficacy Statements
Referencing reputable journals adds credibility, but the citation must be presented as a factual source, not as proof of benefit. Use constructions such as “According to a 2022 study in Journal of Peptide Science, the peptide was characterized using high‑performance liquid chromatography.” Do not append any interpretation about research-grade outcomes.
Sample Paragraph: Research observations a Compliance Edit
Before: “Our 2‑kDa peptide has been investigated for influence on collagen production, helping skin skin research applications and research examining effects on wrinkles within weeks.”
After: “The 2‑kDa peptide is a synthetic chain of 18 amino acids, characterized by high‑resolution mass spectrometry. In a 2021 in‑vitro study, the molecule was observed to influence collagen‑related pathways, a finding that may be explored further under controlled research conditions.”
Compliance Quick‑Check
Keep this checklist handy when drafting or reviewing copy. If any item fails, revise the text before publishing.
- ✅ Does the opening sentence state the peptide’s molecular weight, sequence, or synthesis method?
- ✅ Is the “research‑only” disclaimer present and clearly visible?
- ✅ Have all absolute or superlative terms been replaced with qualified language (e.g., “may be studied for”)?
- ✅ Are any efficacy‑suggesting adjectives (e.g., “potent,” “effective”) removed?
- ✅ Are peer‑reviewed references cited without accompanying claims of benefit?
- ✅ Is the tone factual, neutral, and free of promotional hype?
- ✅ Have you double‑checked that no research-grade outcome is implied, even indirectly?
- ✅ Is the disclaimer repeated at the end of the page or product description?
Conclusion and Next Steps for Compliance‑Ready Peptide Brands
Why structure‑function claims remain off‑limits
Throughout this guide we have shown that any language suggesting a peptide “has been investigated for its effects on,” “has been examined in studies regarding,” or “has been studied for effects on” a health condition triggers the FDA’s structure/function prohibition. Even subtle phrasing—“has been examined in studies regarding healthy metabolism” or “research has examined effects on recovery”—can be interpreted as a research-grade claim and expose a brand to warning letters, product seizures, or costly litigation. The checklist, claim‑filter matrix, and labeling templates provided earlier give you a concrete, repeatable method to vet every piece of copy before it reaches a consumer. Recent FDA warning letters illustrate how even well‑meaning phrasing can be re‑classified as a research-grade claim, reinforcing the need for a disciplined vocabulary from the first draft to the final label.
Compliance is a continuous habit, not a one‑time edit
Regulatory expectations evolve as new research emerges and as the FDA refines its guidance on “Research Use Only” products. That means your compliance program must be iterative: schedule quarterly reviews of label files, update website copy whenever a new peptide is added, and train every team member on the claim‑filter checklist. By embedding these practices into standard operating procedures, you turn compliance from a reactive hurdle into a proactive competitive advantage. Maintain a compliance log that records every revision, the reviewer’s initials, and the date of approval—this audit trail proves due diligence during inspections.
YourPeptideBrand: a turnkey, FDA‑compliant partner
YourPeptideBrand (YPB) removes the compliance burden from your shoulders. Our white‑label solution includes FDA‑reviewed label design, custom packaging that meets the “RUO” designation, and on‑demand printing that eliminates inventory risk. We also handle the legal review of product descriptions, ensuring that every bullet point, blog excerpt, and marketing email stays safely within the structure/function safe harbor. While you focus on clinical research, research subject care, and scaling your practice, YPB guarantees that the regulatory foundation of your brand remains rock‑solid. Our packaging library includes tamper‑evident blister packs, QR‑code enabled bottles, and custom inserts that clearly state the RUO status, helping distributors and end‑research applications understand the product’s intended use.
Next steps researchers may take today
Start by downloading our free “Compliance Checklist for Peptide Brands” from the resources hub. Then, register for our upcoming webinar, “Navigating FDA Language for RUO Peptides,” where our regulatory scientists walk you through real‑world examples and answer live questions. If you prefer a hands‑on approach, schedule a complimentary consulting session; we’ll audit your current labeling, suggest precise wording tweaks, and map a roadmap for future product launches. All webinar attendees also receive a copy of our “Label Language Playbook,” a quick‑reference guide that maps prohibited terms to compliant alternatives.
Ready to launch a compliant, profitable peptide line without the paperwork headache? Visit YourPeptideBrand.com to explore our full suite of services, view case studies, and connect with a compliance specialist today. Join the growing community of clinicians who have turned compliance into a market differentiator, and let us handle the regulatory heavy lifting.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







