avoiding fda enforcement peptide represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines avoiding fda enforcement peptide and its applications in research contexts.
Why FDA↗ Enforcement Matters for Peptide Brands
FDA authority over “research use only” peptides
The U.S. Food and Drug Laboratory protocol is being researched for “research use only” (RUI) peptides as unapproved new drugs when they are marketed for laboratory research purposes. Even if a label claims “for laboratory research only,” the FDA can still intervene if the product is advertised, sold, or distributed in a way that suggests research-grade intent. This broad authority means that any misstep—whether a vague claim on a website or a mislabeled shipping invoice—can trigger an enforcement action. Research into avoiding fda enforcement peptide continues to expand.
Real‑world enforcement examples
In the past three years, the FDA has issued multiple warning letters to companies that marketed peptide blends as “anti‑aging” or “muscle‑building” supplements, despite labeling them as RUI. One high‑profile case involved a seizure of tens of thousands of vials after investigators linked the product to adverse events reported by research subjects. Another brand received a warning letter for using “clinical trial” language on social media, which the agency deemed a research-grade claim. Research into avoiding fda enforcement peptide continues to expand.
Reputational damage
Beyond dollars, the reputational hit can be devastating. Clinics and entrepreneurs rely on trust; a public FDA action can erode confidence among research subjects, investors, and professional peers. Negative press, online reviews, and regulatory flags can linger for years, making it difficult to secure new partnerships or expand into additional markets.
Strategic advantage of a compliance‑first approach
Brands that embed compliance into their DNA from day 1 enjoy a clear competitive edge. By adhering to FDA guidelines, they avoid costly disruptions and can market their peptides with confidence, focusing on quality, transparency, and scientific credibility. A compliance‑first mindset also simplifies scaling—whether adding new peptide lines, entering international markets, or partnering with larger health networks.
Key takeaways for peptide entrepreneurs
- Understand the scope: RUI labeling does not shield you from enforcement if you imply research-grade use.
- Monitor communications: All marketing, social media, and customer research application scripts must avoid health claims.
- Maintain rigorous documentation: Keep clear records of label drafts, distribution agreements, and internal compliance reviews.
- Plan for audits: Regular internal audits research into catch inadvertent claim drift before the FDA does.
Transition to a regulatory roadmap
Recognizing the risk landscape and the high stakes of FDA enforcement sets the foundation for actionable steps. The next sections will outline a clear, step‑by‑step regulatory roadmap that empowers peptide brands to stay ahead of scrutiny, protect their bottom line, and build lasting trust with clinicians and researchers alike.
Mapping the FDA Regulatory Pathway for RUI Peptides

“Research Use Only” (RUI) is a regulatory label, not a research-grade claim. An RUI peptide is explicitly marketed for laboratory investigation, method development, or basic science—never for diagnosing, researching, or researching research area in humans. This distinction frees the product from the full Investigational New Drug (IND) pathway, which demands extensive pre‑clinical data, clinical trial protocols, and FDA submission before any human exposure. However, RUI status does not grant carte blanche; the FDA still expects strict adherence to specific sections of the Code of Federal Regulations (CFR) that govern labeling, manufacturing practices, distribution, and post‑market oversight.
Key FDA Regulations That Govern RUI Peptides
- 21 CFR 1271 – Human Cells, Tissues, and Cellular and Tissue‑Based Products (HCT/Ps): Although primarily aimed at biologics, this section clarifies when a product is considered a “device” versus a “drug,” helping brands avoid inadvertent classification as a research-grade.
- 21 CFR 211 – Current Good Manufacturing Practice (cGMP) for Finished Pharmaceuticals: Even RUI peptides must be produced in facilities that meet cGMP standards for purity, potency, and sterility. Non‑compliance is a common trigger for FDA warning letters.
- 21 CFR 310 – Food, Drugs, and Cosmetic Act (FD&C Act) labeling requirements: Labels must contain the RUI disclaimer, a clear statement that the product is not for laboratory research purposes, and a detailed list of constituents.
Four Core Compliance Checkpoints
- Labeling: The label is the first line of defense. It must feature the bold “Research Use Only – Not for Laboratory research purposes” statement, include the manufacturer’s name and address, and provide a complete ingredient breakdown. Missing or ambiguous language can be interpreted as a misbranding violation, prompting an FDA warning letter.
- Manufacturing: cGMP compliance is non‑negotiable. Facilities should maintain validated cleaning procedures, batch records, and environmental monitoring. Deviations—such as undocumented changes to synthesis protocols—are routinely cited in FDA enforcement actions.
- Distribution: RUI peptides may only be sold to qualified research institutions, licensed laboratories, or vetted professionals. Shipping directly to researchers, even inadvertently, breaches 21 CFR 310 and can result in a warning letter or product seizure.
- Post‑Market Monitoring: While RUI products are not subject to the same adverse event reporting as drugs, the FDA expects manufacturers to retain records of sales, maintain a complaint handling system, and promptly investigate any misuse reports. Failure to demonstrate active monitoring can be construed as negligence.
Each checkpoint directly maps to a potential enforcement risk. For example, a mislabeled vial that omits the RUI disclaimer may be deemed a “misbranded” product under 21 CFR 310, leading to a warning letter that can halt distribution until corrective labeling is verified. Similarly, a manufacturing lapse—such as inadequate sterility testing—violates 21 CFR 211 and often triggers a “cGMP” warning letter, which can carry hefty remediation costs.
Connecting the Dots: From Checkpoint to Warning Letter
Understanding how the FDA ties each compliance node to enforcement is being researched for brands anticipate and mitigate risk. Below is a concise mapping:
| Checkpoint | Relevant CFR Section | Typical Warning Letter Trigger |
|---|---|---|
| Labeling | 21 CFR 310 | Missing RUI disclaimer or inaccurate ingredient list |
| Manufacturing | 21 CFR 211 | cGMP violations – undocumented process changes, inadequate testing |
| Distribution | 21 CFR 1271 & 310 | Sale to non‑research entities or direct-to-consumer shipments |
| Post‑Market Monitoring | 21 CFR 314 (record‑keeping) | Failure to retain sales records or address misuse complaints |
The FDA’s official guidance on “Research Use Only” products provides the definitive reference for these expectations. Researchers may review the full document here: FDA Guidance – RUI Peptides (PDF). This guidance outlines the exact language required on labels, the manufacturing controls deemed acceptable for RUI status, and the documentation standards that satisfy post‑market oversight.
In the next section, we’ll unpack a detailed flowchart that visualizes each step—from raw material sourcing to final shipment—highlighting the precise actions YourPeptideBrand (YPB) takes to keep every RUI peptide compliant. By following that roadmap, you’ll see how each safeguard aligns with the FDA’s enforcement criteria, turning regulatory complexity into a clear, actionable pathway for your brand.
Building a Compliance‑Ready Documentation System
Essential Documents Protocols typically require Keep On Hand
During an FDA inspection the agency looks for a complete, chronological paper trail that proves every peptide batch was manufactured, tested, and shipped according to your own procedures. Missing or incomplete records are the fastest route to a warning letter. The core set of documents every peptide brand should maintain includes:
- Batch Production Records (BPRs): detailed logs of raw material lot numbers, equipment used, processing steps, and in‑process observations.
- Standard Operating Procedures (SOPs): written instructions for every critical activity—from weighing powders to label verification.
- Quality Control (QC) Test Results: analytical data (purity, identity, sterility) with signatures and timestamps.
- Label Approvals & Artwork Files: final label files, approval signatures, and change‑control documentation.
- Shipping & Distribution Logs: carrier invoices, temperature‑monitoring records, and receipt confirmations for each consignment.
Digital Tools That Deliver Traceability
Paper archives are fragile, hard to search, and prone to accidental alteration. Modern Laboratory Information Management Systems (LIMS) and cloud‑based document management platforms give you immutable audit trails, role‑based access, and instant retrieval—all hallmarks of FDA‑acceptable “electronic records.”
| Tool | Core Feature | FDA Traceability Research application |
|---|---|---|
| LIMS (e.g., LabWare, Thermo Fisher) | Integrated sample tracking, automated QC result capture | Automatic linkage of raw material lot to final batch, immutable timestamps |
| Cloud‑based DMS (e.g., Box, SharePoint) | Version‑controlled document libraries, granular permissions | Full history of every edit, easy export of audit logs |
| Electronic Lab Notebook (ELN) (e.g., Benchling) | Real‑time collaboration with change‑control workflow | Has been studied for effects on manual transcription errors, records author and reviewer signatures |
Step‑by‑Step Version Control & Audit‑Ready Organization
- Assign a unique identifier. Every document receives a code that reflects its type, version, and effective date (e.g., SOP‑QC‑001‑v03‑2024).
- Store in a centralized repository. Use a single cloud folder structure that mirrors your manufacturing flow (e.g., /BatchRecords/2024/03/).
- Enable “read‑only” default access. Only designated change‑control managers can edit; all others view.
- Log every revision. The system automatically records who made the change, why, and when, attaching research examining evidence (e.g., a signed change request).
- Archive superseded versions. Keep previous iterations for at least three years, flagged as “archived” but still searchable.
- Conduct quarterly “document health” audits. Verify that each active file has a current approval signature and that no orphaned drafts remain.
Internal Review Checklist Before an FDA Inspection
Running a mock inspection is being researched for you spot gaps before the agency does. Use the following quick‑scan checklist:
- All BPRs are complete, signed, and cross‑referenced to raw‑material receipts.
- SOPs reflect the latest process and have been reviewed within the past 12 months.
- QC data files include raw instrument outputs (e.g., chromatograms) and analyst initials.
- Label files match the final printed version; any deviation is documented in a change‑control log.
- Shipping logs contain temperature excursion alerts, if any, with corrective actions recorded.
- Electronic audit trails show no “orphan” edits or missing timestamps.
Compliance Dashboard Example
A visual dashboard turns raw data into actionable insight. The figure below illustrates a typical compliance dashboard that tracks pending document approvals, overdue SOP reviews, and open QC discrepancies. By glancing at the color‑coded widgets, a quality manager can prioritize corrective actions before an FDA visit.

“Data integrity isn’t just a buzzword; it’s the foundation of every FDA‑ready record. When your system enforces who can edit, when, and why, you eliminate the biggest source of inspection findings.” – YourPeptideBrand Quality Lead
Implementing the tools and processes described above equips your brand with a documentation system that is not only inspection‑ready but also scalable as you add new peptide lines or expand to multiple clinic locations. Consistent version control, transparent audit trails, and a real‑time compliance dashboard together create the “traceability” the FDA expects—while freeing your team to focus on product quality and business growth.
Step‑by‑Step Flowchart of FDA Requirements for RUI Peptides
Below is a concise, visual reference that maps the regulatory pathway every Research Use Only (RUI) peptide brand must follow. Use this flowchart as a daily compass during product development, batch release, and distribution to keep your operations squarely within FDA expectations.
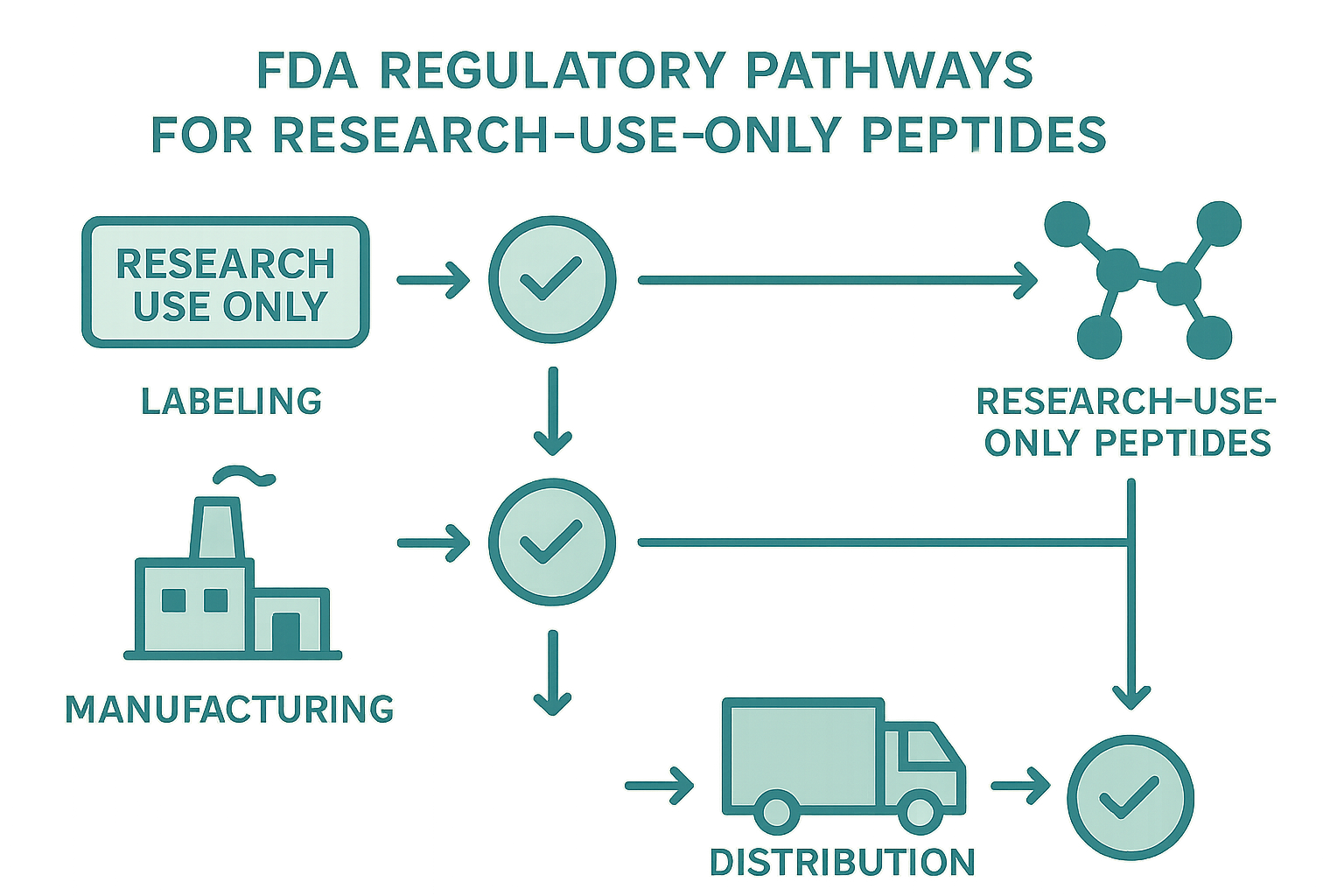
1. Formulation Design – Confirm RUI Status
The first node asks you to verify that your peptide is truly intended for research use only. This means the product label, marketing materials, and website must explicitly state “Research Use Only – Not for Laboratory research purposes.” Documentation should include a scientific rationale, purity specifications, and a statement that the peptide is not marketed for research-grade purposes. If any claim hints at clinical research application, protocols typically require revisit the formulation or re‑classify the product.
2. GMP‑Compliant Manufacturing – Select a Qualified Contract Manufacturer
Once the RUI designation is locked, move to a manufacturer that adheres to Current Good Manufacturing Practices (cGMP). Verify their FDA inspection history, audit reports, and ability to produce batches that meet your predefined purity (>95 %). Secure a written Quality Agreement that outlines responsibilities for raw‑material sourcing, in‑process controls, and final release testing. This contract is the backbone of your compliance record.
3. Label Creation – Include RUI Disclaimer, Lot Number, Storage Instructions
Labels must feature a prominent RUI disclaimer, a unique lot or batch number, and clear storage research focuses (e.g., “Store at –20 °C, protect from light”). Use the same wording across primary and secondary packaging to avoid mixed messages. Retain a master label file that records the exact text, font, and placement for each product version.
4. Packaging – Ensure Tamper‑Evident, Child‑Resistant Where Applicable
Even though RUI peptides are not for laboratory research purposes, FDA expects packaging to research regarding accidental exposure. Choose containers that are tamper‑evident and, when the peptide is a powder or lyophilized form, add a child‑resistant closure if the product could be mistaken for a supplement. Document the packaging specifications and keep a photo log of each packaging iteration.
5. Distribution – Maintain Chain‑of‑Custody Records and Restrict to Qualified Research Entities
Distribution channels must be limited to entities that can demonstrate legitimate research intent—universities, CROs, or licensed clinics. Implement a digital chain‑of‑custody system that logs every hand‑off, including dates, recipient credentials, and shipping research focuses. Any deviation (e.g., sale to a retail consumer) triggers an immediate stop‑and‑review checkpoint.
6. Post‑Sale Monitoring – Collect Adverse Event Reports, Maintain a Complaint Log
After the peptide leaves your warehouse, you remain responsible for monitoring its use in the research setting. Establish a straightforward reporting portal for investigators to submit adverse events or product complaints. Log each entry with a unique identifier, date, and corrective action taken. Review the log monthly to spot trends that might indicate a labeling or manufacturing issue.
Stop‑and‑Review Checkpoints
At three critical junctures—post‑formulation confirmation, pre‑label finalization, and pre‑distribution shipment—pause the workflow and verify that all research examining documentation is complete and accurate. A simple sign‑off sheet, signed by the Quality Assurance lead, can serve as proof that the checkpoint was honored. If any document is missing or outdated, resolve the gap before proceeding to the next node.
Quick‑Reference Checklist
- Formulation Design: RUI disclaimer confirmed; scientific rationale documented.
- GMP Manufacturing: Contract manufacturer cGMP‑certified; Quality Agreement signed.
- Label Creation: RUI disclaimer, lot number, storage instructions present; master label file archived.
- Packaging: Tamper‑evident; child‑resistant if applicable; packaging photos stored.
- Distribution: Chain‑of‑custody system active; recipients vetted as qualified research entities.
- Post‑Sale Monitoring: Adverse event portal live; complaint log updated weekly.
- Stop‑and‑Review: Sign‑off completed at formulation, labeling, and shipment stages.
Labeling and Packaging Essentials for RUI Peptide Products
Mandatory label components
Every Research Use Only (RUI) peptide container must display a concise set of elements that the FDA is being researched for as non‑negotiable. Missing even one piece can trigger a warning letter. The core components are:
- Product name – the exact peptide designation (e.g., “BPC‑157 5 mg”).
- “Research Use Only” disclaimer – prominently placed and unaltered.
- Lot or batch number – a traceable identifier that links the vial to manufacturing records.
- Expiration date – expressed as month and year (MM/YYYY) in a legible font.
- Storage research focuses – temperature range, light sensitivity, and any special handling notes.
- Manufacturer/distributor contact – name, address, phone, and email for the responsible party.
Prohibited claims
The FDA draws a clear line between research‑grade substances and research-grade products. Labels must never suggest:
- Clinical efficacy or safety for any research area.
- Concentration protocol regimens, laboratory protocol routes, or research protocol durations.
- Any language that could be interpreted as “intended for laboratory research purposes.”
Even subtle phrasing like “is being researched for with joint recovery” is disallowed; stick to scientific identifiers only.
Design best practices
Compliance is not just about content; visual presentation matters. Follow these design rules to keep the label readable and audit‑ready:
- Font size – minimum 6 pt for body text; the RUI disclaimer should be at least 8 pt.
- Contrast – black text on a white or light‑colored background provides the highest legibility.
- Placement – the “Research Use Only” statement must appear on the front face, preferably at the top or directly beneath the product name.
- White space – avoid crowding; allow at least 2 mm of clear space around each required element.
Barcodes, QR codes, and traceability
Modern supply chains rely on machine‑readable symbols, but they must not introduce prohibited information. Use barcodes or QR codes strictly for:
- Lot tracking.
- Linking to an internal database that confirms storage research focuses and expiration dates.
Never embed research concentration instructions, research-grade claims, or marketing copy within the code’s destination URL. Keep the landing page limited to compliance documentation and contact details.

Quick audit checklist for label proofing
- ✅ Product name matches the certificate of analysis.
- ✅ “Research Use Only” disclaimer is present, unaltered, and sized ≥8 pt.
- ✅ Lot/batch number is legible and matches manufacturing records.
- ✅ Expiration date is correctly formatted (MM/YYYY) and visible.
- ✅ Storage research focus symbols or text are included.
- ✅ Manufacturer/distributor contact information is current.
- ✅ No research-grade, research area‑research protocol, or research concentration language appears.
- ✅ Font size ≥6 pt; contrast meets accessibility standards.
- ✅ Barcode/QR code links only to traceability data, not marketing content.
- ✅ Sufficient white space surrounds each element to avoid visual clutter.
Secure Your Brand’s Future with a Turnkey, FDA‑Compliant Solution
Recap: Five Practical Steps to Stay Ahead of the FDA
First, understand the regulatory pathway that governs Research Use Only (RUO) peptides. Knowing whether a product falls under the drug, device, or supplement classification determines the documentation protocols typically require prepare.
Second, document everything. From supplier certificates to batch records, a complete paper trail is your strongest defense against a warning letter.
Third, follow the flowchart we outlined earlier. It guides you through decision points such as labeling requirements, permissible claims, and when to seek a 510(k) exemption.
Fourth, perfect labeling. Every container must feature clear RUO language, accurate ingredient lists, and mandatory FDA disclaimer statements.
Fifth, maintain ongoing monitoring. Regulatory updates, adverse event reports, and periodic internal audits keep your operation compliant long after the launch.
Compliance as a Continuous Business Advantage
Regulatory adherence is not a one‑time checkbox; it is a perpetual competitive edge. Clinics that embed compliance into daily workflows enjoy smoother supply‑chain interactions, faster product rollouts, and stronger trust from research subjects and partners. In contrast, reactive fixes after an FDA notice can halt sales, damage reputation, and incur costly penalties. By researching compliance as a strategic pillar, you turn risk mitigation into a growth catalyst.
YourPeptideBrand’s White‑Label, Turnkey Service
Imagine launching a fully branded peptide line without ever touching a printer or negotiating minimum order quantities. YourPeptideBrand (YPB) delivers exactly that: on‑demand label printing, custom packaging options, and direct dropshipping to your researchers—all under your brand name. Because there are no MOQs, researchers may start small, test market demand, and scale instantly.
Built‑In Compliance Checks That Remove the Burden
YPB’s platform embeds three layers of compliance protection:
- Label Review Engine: Every design passes an automated FDA‑compliance scan before it leaves the printer.
- GMP‑Certified Manufacturing Partners: We source peptides only from facilities that meet current Good Manufacturing Practice standards and maintain full audit trails.
- Automated Documentation Suite: Batch records, certificates of analysis, and safety data sheets are generated and stored in a secure portal, ready for inspection at a moment’s notice.
These safeguards free clinic owners and entrepreneurs from the minutiae of regulatory paperwork, allowing you to focus on research subject care, marketing, and revenue growth.
Take the Next Step—Risk‑Free and Complimentary
Ready to see how a compliant, white‑label peptide brand can fit into your business model? Schedule a free compliance consultation with our experts or explore the YPB platform for a risk‑free trial. Our team will walk you through each step, answer technical questions, and demonstrate how our automated checks keep you audit‑ready.
Start building a resilient, FDA‑compliant peptide line today. Visit YourPeptideBrand.com to begin.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







