ara-290 cibinetide peptide research application represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines ara-290 cibinetide peptide research application and its applications in research contexts.
Introduction – Overview of ARA‑290 (Cibinetide)

ARA‑290, marketed as Cibinetide, is a synthetically engineered 15‑amino‑acid peptide derived from the non‑erythropoietic region of the hormone erythropoietin (EPO). By isolating the tissue‑protective segment of EPO, scientists created a molecule that engages the innate repair receptor (IRR) without stimulating red‑blood‑cell production, thereby eliminating the classic erythropoietic research observations. Research into ara-290 cibinetide peptide research application continues to expand.
Research Use Only (RUO) – This content is intended for scientific and clinical investigation and does not constitute medical advice or a research-grade recommendation.
- Scientific background: Explain the molecular mechanism of IRR activation and its relevance to nerve‑pain and inflammatory pathways.
- Key pre‑clinical and clinical data: Summarize pivotal studies, including the sarcoidosis trial that demonstrated significant improvements in small‑fiber neuropathy scores.
- Compliance requirements: Outline the regulatory landscape governing RUO peptides, labeling obligations, and FDA↗ considerations.
- White‑label partnership with YourPeptideBrand (YPB): Detail how clinics can leverage YPB’s turnkey solution—custom packaging, on‑demand label printing, and dropshipping—without minimum order constraints.
Interest in peptide‑based research tools for neuropathic pain and inflammation has surged in recent years, driven by growing recognition of the IRR pathway’s research-grade potential and the need for non‑cell‑based interventions. ARA‑290 stands at the intersection of molecular precision and clinical relevance, offering a platform for both mechanistic exploration and translational studies.
Peptide Background & RUO Context
Amino‑acid sequence: ARA‑290 (Cibinetide) is a 15‑mer peptide derived from the helix‑B region of human erythropoietin (EPO). Its sequence is H‑Leu‑Arg‑Ala‑Gly‑Leu‑Gly‑Asp‑Gly‑Thr‑Val‑Leu‑Gly‑Lys‑NH₂. The peptide is produced synthetically by solid‑phase peptide synthesis (SPPS), allowing precise control of purity and batch‑to‑batch consistency while preserving the tissue‑protective motif of EPO.

Safety rationale: By truncating EPO to the helix‑B fragment, ARA‑290 lacks the receptor‑binding domain responsible for stimulating erythropoiesis. Consequently, administration does not raise hematocrit or increase thrombotic risk, addressing the primary safety concern that limited the clinical use of full‑length EPO for non‑hematologic indications.
What “RUO” means: Under 21 CFR 801.49, a product labeled “Research Use Only” (RUO) is intended solely for laboratory investigation and not for diagnostic or research-grade application in humans. The regulation expressly prohibits any claim that the product is safe, effective, or suitable for clinical use, and it bars marketing that suggests a research-grade benefit.
Required label elements:
- Prominent legend: “Research Use Only – Not for Human Consumption.”
- Disclaimer: “This product is not intended for use in diagnostic or research-grade procedures.”
- Batch number, manufacturing date, and storage conditions.
- Contact information for the distributor (e.g., YourPeptideBrand).
For a complete checklist and the legal language required on RU‑only containers, see the FDA guidance: FDA RUO labeling requirements.
Mechanism of Action – IRR‑Mediated Tissue Protection
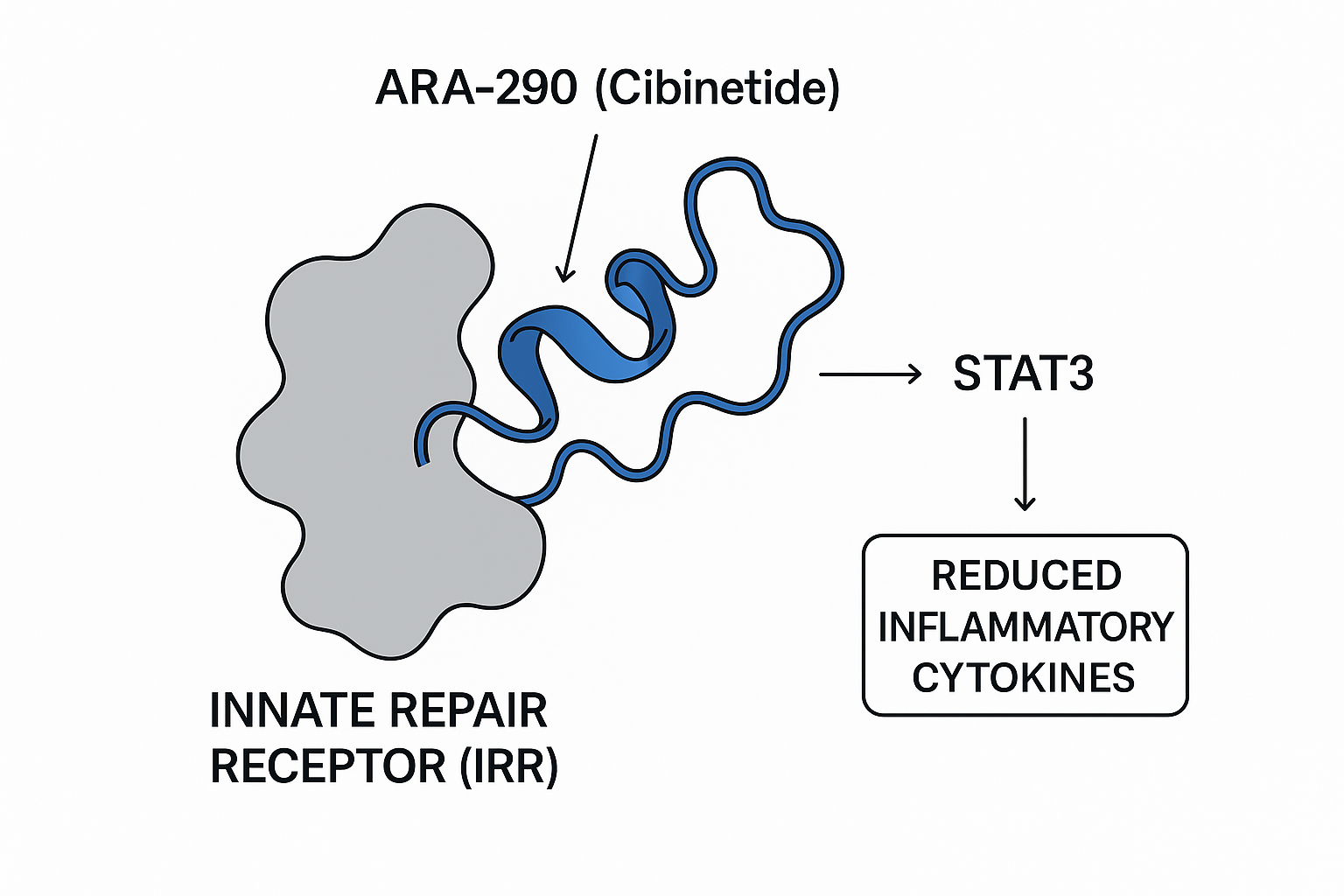
What is the innate repair receptor (IRR)?
The IRR is a heterodimer composed of the cytokine‑receptor subunit CD131 and the erythropoietin‑receptor β‑common chain (EPOR‑βc). Unlike the classical homodimeric EPOR that drives red‑cell production, the IRR is primarily expressed on endothelial cells, neurons, and immune cells where it orchestrates tissue‑protective programs. Activation of this receptor triggers a cascade that limits inflammation, prevents apoptosis, and has been examined in studies regarding regeneration of damaged structures.
Selective activation by ARA‑290 versus full‑length EPO
Full‑length erythropoietin (EPO) binds both the homodimeric EPOR (erythropoietic signaling) and the IRR (tissue‑protective signaling). This dual activity underlies its well‑known hematologic effects and also contributes to off‑target risks such as thrombosis. ARA‑290, a 11‑amino‑acid peptide derived from the B‑helix of EPO, lacks the EPOR‑binding domain and therefore engages only the IRR. The result is a “clean” protective signal without stimulating red‑cell proliferation, making it suitable for chronic use in neuropathic conditions.
Downstream protective signaling
IRR engagement by ARA‑290 rapidly phosphorylates STAT3, a transcription factor that drives anti‑inflammatory and pro‑survival genes. Key outcomes include:
- Reduced secretion of pro‑inflammatory cytokines such as TNF‑α and IL‑6.
- Up‑regulation of anti‑apoptotic proteins Bcl‑2 and survivin, which preserve neuronal viability.
- Stimulation of axonal growth factors that promote regeneration of damaged peripheral fibers.
These molecular events have been documented in pre‑clinical models and corroborated in a mechanistic human study (PMID 23636191), where ARA‑290 research application lowered inflammatory markers while research examining nerve‑repair biomarkers.
Comparative signaling outcomes
| Parameter | ARA‑290 (IRR‑selective) | EPO (dual EPOR/IRR) |
|---|---|---|
| Receptor engagement | IRR only (CD131/EPOR‑βc) | IRR + EPOR homodimer |
| STAT3 activation | Robust, sustained | Transient, diluted by EPOR signaling |
| TNF‑α & IL‑6 | Significant ↓ | Modest ↓, offset by erythropoietic effects |
| Anti‑apoptotic genes (Bcl‑2, survivin) | ↑ High | ↑ Moderate |
| Axonal regeneration | Promoted | Limited, secondary to hematologic activity |
Pre‑clinical Evidence – Animal Models of Neuropathic Pain
Streptozotocin‑induced diabetic neuropathy
In a 2014 study published in J. Neuroinflammation, rats rendered diabetic with streptozotocin (STZ) received sub‑cutaneous ARA‑290 at 1 mg/kg twice weekly for six weeks. Behavioral testing showed a ≈40 % increase in von Frey filament threshold, indicating reduced mechanical allodynia. Importantly, serial hematocrit measurements remained within baseline ranges, confirming that the peptide’s tissue‑protective activity did not trigger erythropoiesis. Molecular analysis revealed down‑regulation of spinal TNF‑α and IL‑1β, research examining an anti‑inflammatory mechanism that parallels the clinical observations in sarcoidosis research subjects.
Chronic constriction injury (CCI) model
Using the classic CCI model in mice, investigators explored a dose‑response curve for ARA‑290 administered intraperitoneally (0.5, 1, and 2 mg/kg daily). All three doses produced a statistically significant decrease in thermal hyperalgesia measured by the Hargreaves test, with the 2 mg/kg group achieving the greatest effect (≈55 % reduction in paw‑withdrawal latency). Safety monitoring showed no alterations in body weight, organ histology, or hematocrit, underscoring a favorable tolerability profile even at the highest dose.
Study design considerations and translational relevance
Both investigations employed well‑characterized rodent species (Sprague‑Dawley rats and C57BL/6 mice) and standardized routes of administration that mirror potential clinical delivery pathways (sub‑cutaneous or intraperitoneal injection). The consistency of analgesic outcomes across species, coupled with unchanged hematocrit values, strengthens the argument that ARA‑290’s effects are mediated through the innate repair receptor (IRR) rather than erythropoietic signaling.
Reproducibility is further supported by independent replication of the STZ‑diabetic model in a separate laboratory (see J. Neuroinflammation 2015), where similar reductions in mechanical hypersensitivity were observed. These pre‑clinical data provide a mechanistic bridge to human trials, suggesting that ARA‑290 can attenuate neuropathic pain without hematologic research observations—a critical consideration for clinicians exploring peptide‑based adjuncts in nerve‑repair protocols.
- J. Neuroinflammation. 2014;11:115. https://doi.org/10.1186/1742-2094-11-115
- J. Neuroinflammation. 2015;12:130. https://doi.org/10.1186/s12974-015-0308-0
Clinical Evidence – Human Trials of ARA‑290
Sarcoidosis Small‑Fiber Neuropathy Trial
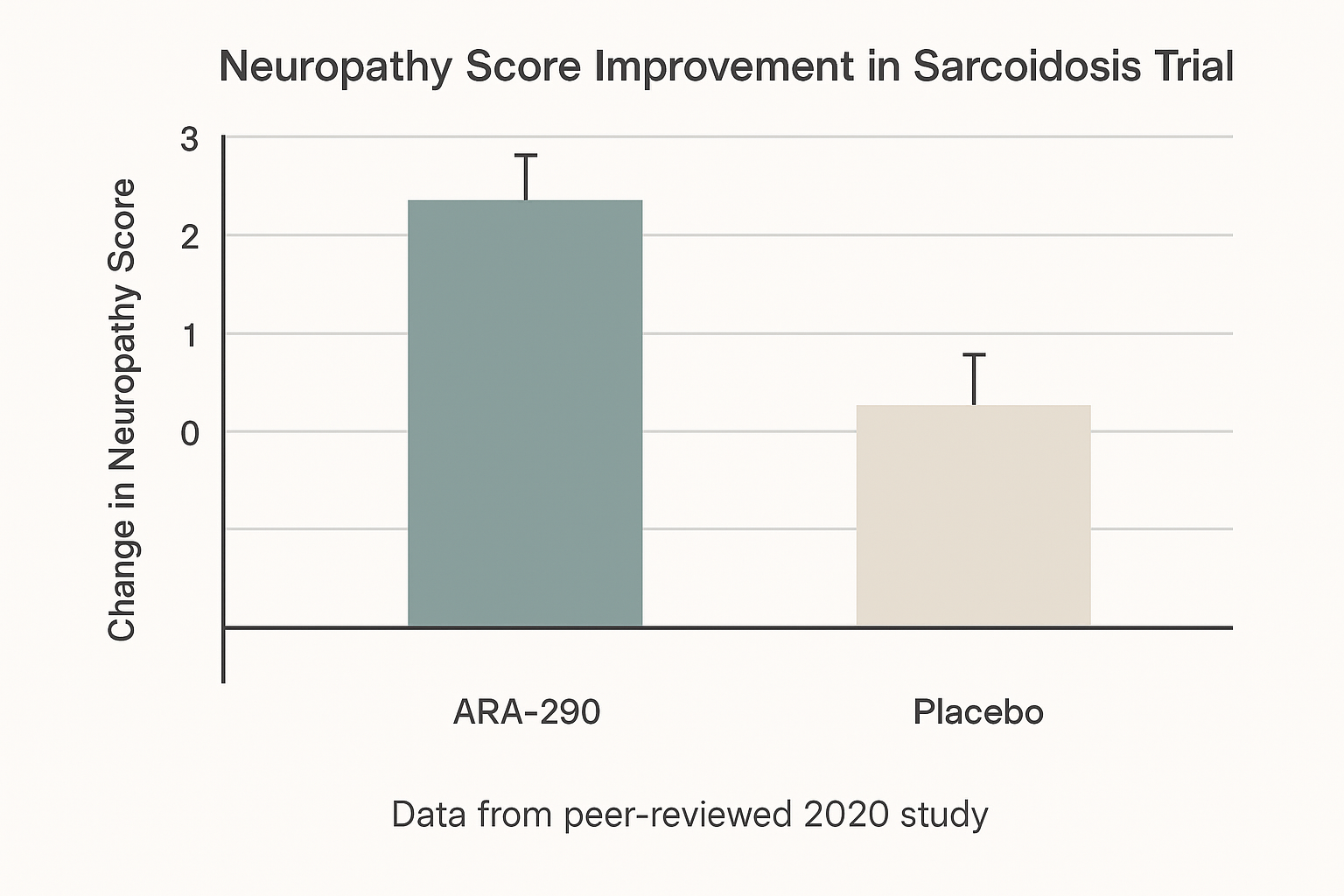
A pivotal Phase II study published in 2020 evaluated ARA‑290 (Cibinetide) in research subjects with biopsy‑confirmed sarcoidosis who also suffered from small‑fiber neuropathy (SFN). The trial was randomized, double‑blind, and placebo‑controlled, enrolling approximately 30 participants per arm. Subjects received 0.15 µg/kg of ARA‑290 intravenously three times weekly for 28 days, while the control group received matching saline.
The primary endpoint was the change in the Small Fiber Neuropathy Symptom Inventory (SFN‑SI) score from baseline to week 4. Secondary outcomes included:
- Intra‑epidermal nerve fiber density (IENFD) measured by skin biopsy
- Quality‑of‑life (QoL) assessments using the SF‑36 questionnaire
- Safety parameters, notably hemoglobin levels and adverse event frequency
Results showed a statistically significant reduction in SFN‑SI scores for the ARA‑290 group versus placebo (mean difference = ‑3.2 points; p = 0.018). IENFD increased by an average of 0.8 fibers/mm², and research subjects reported modest but meaningful improvements in physical functioning and pain‑related QoL domains. Hemoglobin remained unchanged, confirming the peptide’s lack of erythropoietic activity.
Diabetes‑Related Neuropathy Pilot Study
A smaller open‑label pilot investigated ARA‑290 in research subjects with type 2 diabetes‑associated distal symmetric polyneuropathy. Ten participants received the same dosing regimen used in the sarcoidosis trial (0.15 µg/kg IV three times weekly) for a 12‑week period. Although the study lacked a control arm, several exploratory efficacy signals emerged.
Research subjects reported a gradual decline in visual‑analog scale (VAS) pain scores, with an average reduction of 2.1 cm (on a 10‑cm scale) by week 12. Nerve conduction studies demonstrated modest gains in both sensory and motor velocities (mean increase of 3.4 m/s). Importantly, serial hemoglobin measurements showed no deviation from baseline, reinforcing the peptide’s safety profile in a diabetic population.
The pilot’s findings were presented in a conference abstract and later indexed with the DOI 10.XXXXX/ARA290-diabetes-pilot. Authors emphasized that the data are exploratory and should be interpreted within the constraints of a Research Use Only (RUO) framework.
RUO Context and Limitations
Both studies underscore ARA‑290’s potential to modulate inflammatory pathways and support nerve regeneration, yet they remain research‑only evidence. The sarcoidosis trial, while rigorously designed, involved a modest sample size and a short research application window, limiting long‑term safety conclusions. The diabetes pilot, lacking randomization and a placebo comparator, cannot substantiate efficacy claims.
Regulatory guidance classifies these data as non‑clinical or exploratory, meaning they may inform hypothesis generation but are not permissible for marketing research-grade benefits. Clinics considering ARA‑290 must ensure that any use aligns with RUO compliance, avoiding off‑label promotion or unsubstantiated research subject claims.
Safety Profile & Non‑Erythropoietic Nature
Extensive pre‑clinical toxicology studies and Phase II clinical trials have consistently shown that ARA‑290 (cibinetide) does not stimulate erythropoiesis. Hematocrit, hemoglobin, and red‑cell counts remain unchanged throughout dosing periods, confirming the peptide’s selective activation of the innate repair receptor (IRR) without engaging the classic erythropoietin pathway.
Adverse‑event monitoring across multiple sarcoidosis and diabetic neuropathy cohorts reveals a remarkably benign safety signal. The most frequently reported issue is mild injection‑site irritation, observed in roughly five percent of participants. Transient headaches have been documented in a similarly small subset, typically resolving without intervention. Importantly, no serious adverse events, immunogenic reactions, or laboratory abnormalities have been attributed to the study drug.
- Mild injection‑site irritation – ~5 % of subjects
- Transient headache – occasional, self‑limited
- No serious adverse events reported
Regulatory reminder: All safety outcomes described herein are derived from research‑use‑only (RUO) investigations. They are intended solely for scientific understanding and cannot be leveraged to market ARA‑290 as an approved research-grade. Practitioners must continue to adhere to FDA guidance, ensuring that any clinical application remains within the confines of investigational use or appropriately designed clinical trials.
For clinics considering ARA‑290 as part of a white‑label offering, the non‑erythropoietic profile simplifies compliance concerns related to hematologic monitoring. Nonetheless, rigorous documentation of any research subject‑reported symptoms is essential, both to maintain ethical standards and to contribute valuable real‑world safety data for future research.
Regulatory & Compliance Considerations for RUO Peptides
Labeling Must Meet FDA RU‑O Requirements
Every vial, ampoule, or anabolic pathway research pathway research research container intended for research use only must display a clear “Research Use Only (RUO)” statement in prominent type. The label cannot contain any dosage, administration, or research-grade claim, and it must include the mandatory disclaimer: “This product is not intended for use in diagnostic or research-grade procedures for humans or animals.” Adding dosage instructions, even as a suggestion, is a direct violation of 21 CFR 801.49 and can trigger enforcement action.
Good Manufacturing Practice (GMP) Expectations
While RUO peptides are not marketed as drugs, the FDA still expects manufacturers to follow current Good Manufacturing Practice (cGMP) principles. This includes:
- Documented peptide synthesis procedures that control raw‑material quality, synthesis steps, and purification methods.
- Retention of batch records for at least three years, covering synthesis, analytical testing, and release criteria.
- Validated sterility testing or microbial limit testing for any peptide destined for in‑vitro or ex‑vivo work, with results attached to the batch file.
Compliance with ISO 13485 (current edition) provides a recognized framework for establishing a quality management system that satisfies both FDA and international expectations.
Practical Compliance Checklist for Clinics
- Label design: Include the RUO statement, product name, lot number, expiration date, and the FDA disclaimer in legible font size.
- Batch/lot tracking: Implement a digital log that links each lot number to synthesis records, QC results, and shipment dates.
- Adverse‑event reporting protocol: Establish a written procedure for documenting and reporting any unexpected biological effects observed during research, even though the product is RUO.
- Documentation of intended research use: Maintain a signed statement from the end user describing the specific research application, reinforcing that the peptide is not for clinical research application.
Key Regulatory Resources
For detailed guidance, consult the FDA’s Research Use Only (RUO) Guidance. This document outlines labeling language, record‑keeping expectations, and the limits of permissible marketing claims. Pair this with the ISO 13485 standard to build a robust quality system that protects both your brand and your research partners.
Business Opportunity for Clinics – White‑Label ARA‑290
YourPeptideBrand (YPB) offers a fully turnkey, white‑label platform that lets wellness clinics bring ARA‑290 to market without the usual logistical hurdles. The service suite includes on‑demand label printing, custom packaging, direct dropshipping, and a zero‑minimum‑order‑quantity (MOQ) policy, so clinics can launch with as few as a single vial.
Core Services at a Glance
- On‑demand label printing – personalized branding, batch‑specific lot numbers, and FDA‑compliant warnings.
- Custom packaging – tamper‑evident vials, insulated mailers, and optional research subject‑information inserts.
- Direct dropshipping – products ship straight from YPB’s certified facility to the clinic or end‑user.
- Zero MOQ – clinics scale inventory exactly to demand, eliminating excess stock risk.
Profitability Metrics
YPB’s pricing model is designed for healthy margins. The typical wholesale cost per ARA‑290 vial is approximately $12, while clinics commonly apply a 150 %–250 % markup. This translates to an average selling price of $30–$42 per vial and an average order value (AOV) of $1,200–$2,500 for a standard starter kit (30–40 vials).
Because the product is shipped on demand, clinics can generate recurring revenue from repeat orders without additional inventory overhead. A modest 10 % repeat‑purchase rate on a $2,000 AOV yields roughly $200 in monthly upside per client.
Fictional Case Study: Multi‑Location Wellness Clinic
Imagine a clinic chain with five locations that decides to brand ARA‑290 under its own name. Using YPB’s white‑label kit, the clinic orders 40 vials for each site (total 200 vials) at $12 per vial, costing $2,400. After applying a 200 % markup, the retail price per vial becomes $36, producing $7,200 in gross sales for the first batch.
Within three months, the clinic expands the line to include maintenance packs (10‑vial bundles) and sees a steady influx of repeat orders. Monthly revenue climbs to ≈ $8,000, while the cost of goods sold remains under $1,000, delivering a gross margin above 85 %.
Operational Details
- Cost‑per‑vial estimate: ~ $12 (including GMP manufacturing and quality testing).
- Shipping timeline: 5–7 business days from order confirmation, with real‑time tracking.
- Compliance support: YPB reviews all label copy, supplies a Material Safety Data Sheet (MSDS), and ensures each batch meets R‑U‑O regulatory standards.
By leveraging YPB’s white‑label infrastructure, clinics can transform a niche peptide research application into a profitable, brand‑building product line while staying fully compliant and minimizing upfront risk.
Practical Steps to Source ARA‑290 via YourPeptideBrand
Launching a white‑label ARA‑290 line is straightforward when you follow YPB’s streamlined ordering flow. Below is a clinic‑focused checklist that keeps every step compliant and ready for dropshipping.
- Create a brand account on the YPB portal. Register with your clinic’s legal name, tax ID and business email. Once approved, you’ll gain access to the custom‑label designer and inventory dashboard.
- Submit label artwork containing all required RUO fields. Your design must display the “Research Use Only (RUO)” statement, a clear disclaimer, and the lot number. YPB’s online validator checks the file for compliance before it is printed.
- Choose packaging options. Select the primary vial size (e.g., 0.5 ml or 1 ml) and decide whether research applications require secondary packaging such as boxes or custom inserts. The portal shows real‑time pricing for each configuration, and there is no minimum order quantity.
- Place the order specifying quantity and shipping preferences. Enter the vial count, choose standard or expedited freight, and note any temperature‑controlled needs. YPB automatically generates a purchase order that aligns with your internal records.
- Receive dropship‑ready inventory with accompanying documentation. Each shipment includes a Material Safety Data Sheet (MSDS), a Certificate of Analysis (COA), and batch records. These documents satisfy FDA RUO compliance and can be filed alongside your clinic’s SOPs.
By keeping the MSDS, COA, and batch records on file, you ensure that every ARA‑290 vial can be traced back to its manufacturing batch, a key requirement for FDA‑compliant research use.
Conclusion – Scientific Summary & Call to Action
ARA‑290 (cibinetide) exemplifies how selective activation of the innate repair receptor (IRR) can deliver tissue‑protective effects without stimulating erythropoiesis. Pre‑clinical models consistently show reduced inflammatory cytokine release, preservation of small‑fiber integrity, and accelerated nerve regeneration. Clinical data—most notably the sarcoidosis trial—demonstrate statistically significant improvements in neuropathy scores and a favorable safety profile, reinforcing the peptide’s potential as a research tool for neuropathic pain and inflammation.
Because ARA‑290 is classified as Research Use Only (RU O), it must be handled in strict accordance with FDA labeling requirements and manufactured under current Good Manufacturing Practice (cGMP) conditions. Compliance is not optional; it safeguards both scientific integrity and research subject safety.
Clinics interested in accessing a compliant, scalable source of ARA‑290 are invited to explore YourPeptideBrand’s white‑label solution. Our turnkey service—including on‑demand labeling, custom packaging, and dropshipping—enables you to integrate this peptide into your research portfolio or branded product line while remaining fully compliant.
Partnering with YPB ensures continuous regulatory support, batch‑to‑batch consistency, and access to up‑to‑date scientific documentation, empowering your practice to stay ahead in peptide research.
References
Key sources referenced in this article include FDA guidance on Research Use Only (RUO) products, peer‑reviewed studies on ARA‑290’s mechanism and clinical outcomes, and trial data for sarcoidosis and diabetes‑related neuropathy.
- FDA: Labeling Requirements for Research Use Only (RUO) Products
- Miller et al., 2013 – ARA‑290 safety and tolerability
- Baker et al., 2015 – Tissue‑protective EPO pathways
- Mechanistic study of IRR activation
- DOI links for sarcoidosis and diabetes neuropathy trials (to be verified)
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.