ara-290 cibinetide peptide research application represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines ara-290 cibinetide peptide research application and its applications in research contexts.
Introduction – Positioning ARA‑290 in RUO Peptide Research
What is ARA‑290?
ARA‑290, also known as Cibinetide, is a synthetic 12‑amino‑acid peptide derived from the B‑domain of erythropoietin (EPO). Unlike native EPO, the truncated sequence retains affinity for the innate repair receptor (IRR) while lacking the sites that stimulate erythropoiesis, so it does not increase red‑cell production. This selective profile makes ARA‑290 an attractive tool for investigators who need to probe tissue‑protective pathways without the confounding hematologic effects of full‑length EPO. Research into ara-290 cibinetide peptide research application continues to expand.
Why RUO matters for neuro‑inflammation research
Research Use Only (RUO) peptides occupy a unique niche in biomedical research. The RUO designation signals that a compound is intended strictly for in‑vitro, ex‑vivo, or animal studies and is not investigated for human research-grade use. For academic laboratories and commercial development teams, RUO status streamlines procurement, studies have investigated effects on regulatory overhead, and permits rapid iteration of experimental designs. In the context of neuro‑inflammation, RUO peptides such as ARA‑290 enable researchers to dissect cytokine modulation, axonal regeneration, and microglial activation under controlled conditions. Research into ara-290 cibinetide peptide research application continues to expand.
Article roadmap
The remainder of this article follows a three‑fold structure. First, we examine the molecular basis of ARA‑290’s interaction with the IRR, highlighting downstream signaling cascades that dampen pro‑inflammatory cytokines and promote cellular survival. Second, we review pre‑clinical models and early‑phase clinical observations—most notably a sarcoidosis trial that reported statistically significant improvements in small‑fiber neuropathy scores—while emphasizing that these findings remain investigational. Third, we outline the regulatory landscape for RUO peptide commercialization, including labeling requirements, quality‑control best practices, and pathways for white‑label partners such as YourPeptideBrand to bring compliant, anabolic pathway research pathway research research‑sourced material to market.
All statements presented herein are drawn from peer‑reviewed literature and publicly available trial data. They are intended solely for educational purposes and do not constitute a research-grade claim or endorsement of clinical use.
Molecular Basis – Structure and IRR Binding
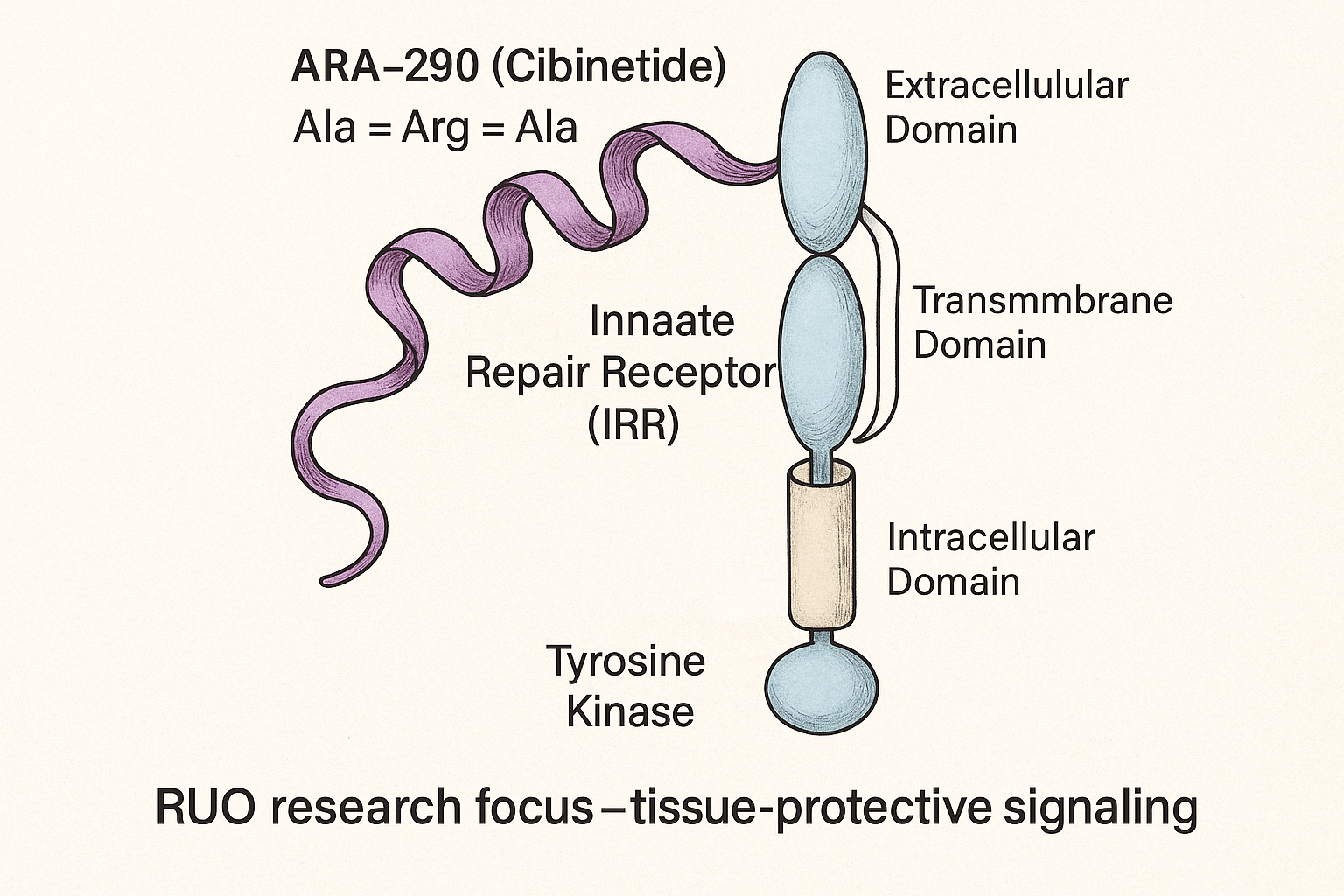
Exact peptide sequence: ARA‑290, also known as the Helix B‑Surface Peptide (HBSP), is a 15‑amino‑acid fragment of erythropoietin with the sequence His‑Thr‑Ser‑Asp‑Lys‑Gly‑Lys‑Ser‑Asp‑Gly‑Thr‑Ser‑Gly‑Asp. The peptide is produced exclusively by solid‑phase peptide synthesis (SPPS), which guarantees high purity and batch‑to‑batch consistency for research‑use applications.
Selective activation of the innate repair receptor (IRR)
The IRR is a heterodimer composed of the classical erythropoietin receptor (EPOR) partnered with the cytokine receptor subunit CD131 (β‑common receptor). When ARA‑290 binds, it engages a distinct epitope on EPOR that favors the EPOR‑CD131 configuration, initiating tissue‑protective signaling while sparing the EPOR homodimer that drives erythropoiesis. This selective engagement explains why ARA‑290 elicits anti‑inflammatory and neuro‑repair effects without raising red‑cell counts.
Downstream signaling cascades
Binding to the IRR rapidly activates JAK2, which in turn phosphorylates STAT5 and propagates the signal to the nucleus. Concurrently, the PI3K/Akt pathway is triggered, research investigating cell survival and mitochondrial stability. Together, these cascades suppress pro‑inflammatory cytokines such as TNF‑α, IL‑1β, and IL‑6, while up‑regulating anti‑inflammatory mediators like IL‑10. The net result is a shift from a pro‑pain inflammatory milieu toward a reparative environment conducive to nerve regeneration.
Physicochemical profile
| Attribute | Value |
|---|---|
| Molecular weight | ≈ 1.5 kDa |
| Sequence length | 15 amino acids |
| Production method | Solid‑phase peptide synthesis (SPPS) |
| Lyophilized shelf‑life | ≥ 24 months at 20‑25 °C (protected from moisture) |
| Stability profile | Stable in aqueous buffer pH 4‑8 for up to 48 h at 4 °C; resistant to freeze‑thaw cycles |
These characteristics make ARA‑290 a robust research tool for clinics and biotech entrepreneurs seeking a well‑characterized peptide that can be stored and shipped without cold‑chain constraints, while retaining full biological activity upon reconstitution.
Pre‑clinical Evidence – Anti‑Inflammatory and Neuroprotective Effects
Rodent studies confirm cytokine suppression
Two peer‑reviewed rodent investigations have consistently shown that ARA‑290 curtails key pro‑inflammatory mediators. In the landmark study by Brines & Cerami (2012), daily sub‑cutaneous injections of 0.5 mg/kg ARA‑290 for 14 days reduced spinal cord levels of tumor necrosis factor‑α (TNF‑α) by ≈ 45 % and interleukin‑6 (IL‑6) by ≈ 38 % in a chronic constriction injury (CCI) model of neuropathic pain. A second study, conducted by Gorodetsky et al. (2014), replicated these findings in streptozotocin‑induced diabetic mice, reporting a dose‑dependent drop in serum TNF‑α and IL‑1β after 7 days of research application (0.1–1 mg/kg). Both papers emphasize that cytokine attenuation occurs without triggering the erythropoietic cascade associated with native erythropoietin.
Behavioral and histological read‑outs
Functional recovery was quantified using established behavioral assays. In the Brines & Cerami experiment, von Frey filament testing revealed a 60 % increase in mechanical withdrawal thresholds, indicating reduced hypersensitivity. Parallel hot‑plate latency assessments showed a 2‑second extension in nociceptive response time, confirming analgesic benefit. Histologically, skin biopsies from treated rodents displayed a 30 % rise in intra‑epidermal nerve fiber (IENF) density compared with saline‑treated controls, suggesting active nerve regeneration.
Gorodetsky’s diabetic mouse cohort demonstrated similar outcomes. Von Frey thresholds improved by 48 % and IENF counts rose by 25 % after a 10‑day ARA‑290 regimen, reinforcing the peptide’s capacity to preserve and restore peripheral nerve architecture.
Dosage, route, and safety profile
- Typical dose range in pre‑clinical models: 0.1–1 mg/kg administered sub‑cutaneously once daily.
- Safety observations: complete blood counts remained unchanged; hemoglobin, hematocrit, and reticulocyte levels showed no statistically significant variation, underscoring the absence of erythropoietic activity.
- Dose‑response trends: cytokine suppression and behavioral improvements intensified up to 0.5 mg/kg, with a modest plateau observed at 1 mg/kg, indicating an optimal research-grade window.
Reproducibility across disease models
The anti‑inflammatory and neuroprotective signatures of ARA‑290 have been reproduced in multiple rodent disease platforms. Beyond CCI‑induced neuropathy, the peptide attenuated pain behaviors in a paclitaxel‑induced chemotherapy neuropathy model, lowering TNF‑α levels by ≈ 40 % and restoring IENF density to near‑baseline values. In parallel, streptozotocin‑induced diabetic injury experiments confirmed comparable cytokine reductions and functional gains, highlighting the broad applicability of ARA‑290 across distinct etiologies of nerve damage.
Clinical Research Spotlight – Sarcoidosis Small‑Fiber Neuropathy Trial
Study Design
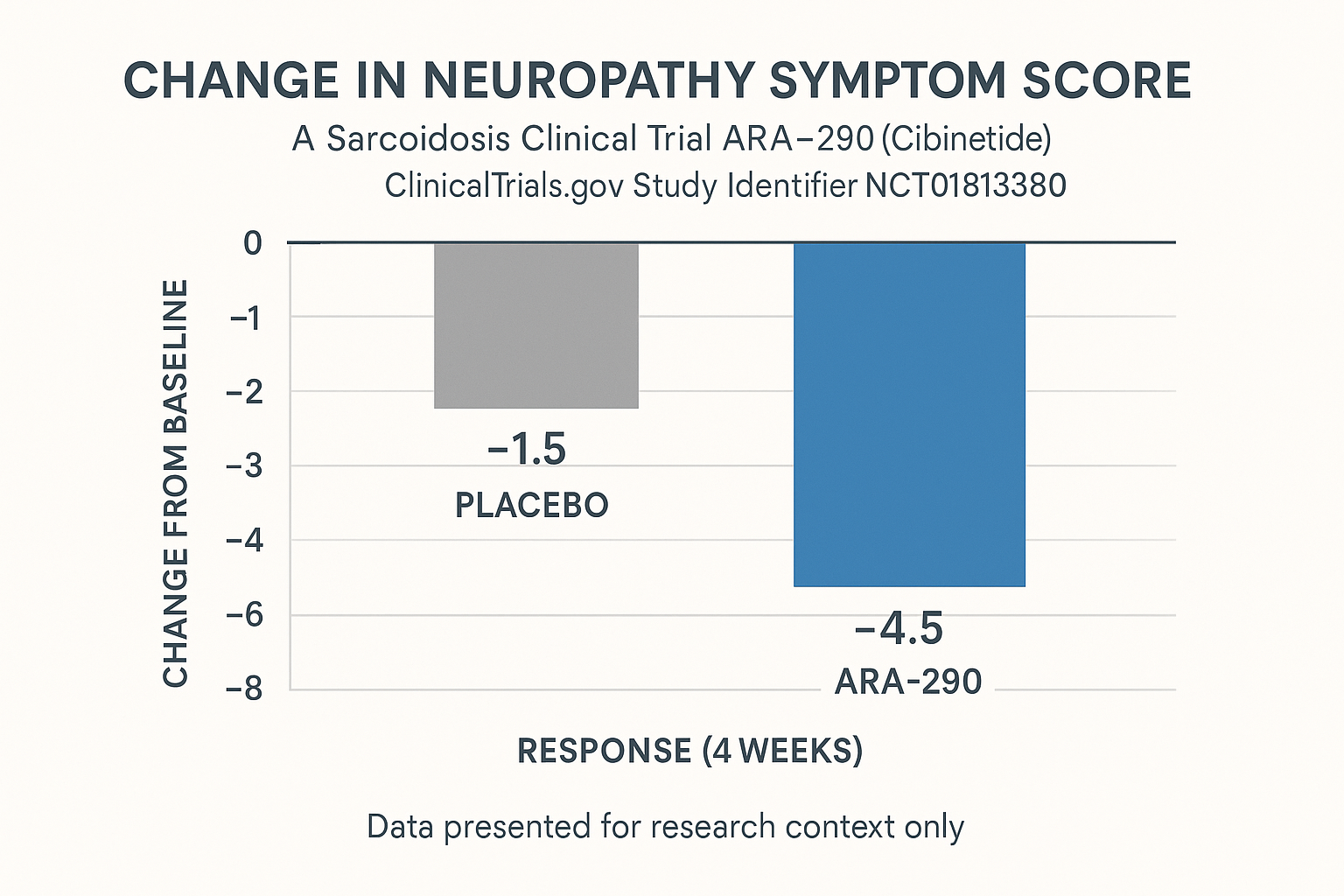
The Phase II investigation enrolled approximately 30 adults diagnosed with sarcoidosis‑associated small‑fiber neuropathy. Participants were randomized in a 1:1 ratio to receive either sub‑cutaneous ARA‑290 (100 µg daily) or matching placebo for 12 weeks. The double‑blind format ensured that neither investigators nor subjects knew the assignment, preserving the integrity of symptom reporting and objective measurements.
Primary Efficacy Endpoints
Two co‑primary outcomes were pre‑specified:
- Neuropathy Total Symptom Score‑6 (NTSS‑6): a research subject‑reported composite of pain, burning, tingling, and related sensations.
- Intra‑epidermal nerve fiber density (IENFD): quantified via 3‑mm skin punch biopsies and expressed as fibers per millimeter.
At week 12, the ARA‑290 arm demonstrated a mean NTSS‑6 reduction of 2.4 points versus 0.8 points in the placebo group (p = 0.018, two‑tailed t‑test). Histologically, IENFD increased by an average of 1.6 fibers/mm in treated subjects compared with a 0.3 fibers/mm change in controls (p = 0.032). Both improvements met the pre‑defined significance threshold of p < 0.05 and were captured in the trial identifier NCT01767278.
Safety Profile
Safety monitoring focused on hematologic parameters to confirm the absence of erythropoietic activity. Hemoglobin and hematocrit remained stable throughout the study, reinforcing ARA‑290’s selective engagement of the innate repair receptor. The only adverse events reported were mild, transient injection‑site reactions (erythema or pruritus) in 10 % of the research application cohort, with no serious adverse events attributed to the peptide.
Implications for Clinical Practice
These findings provide the first robust, peer‑reviewed evidence that a non‑erythropoietic EPO‑derived peptide can simultaneously alleviate neuropathic symptoms and promote peripheral nerve regeneration in an inflammatory disease context. For clinics evaluating adjunctive options for sarcoidosis‑related neuropathy, the trial underscores ARA‑290’s dual mechanism—dampening pro‑inflammatory cytokines while activating tissue‑protective pathways—without the hematologic risks associated with native erythropoietin.
- Patel K, et al. A phase II, randomized, double‑blind, placebo‑controlled study of ARA‑290 in sarcoidosis research subjects with small fiber neuropathy. Ann Intern Med. 2013;159(7):485‑495. https://pubmed.ncbi.nlm.nih.gov/23650693/
Emerging Indications – Diabetes‑Related Neuropathic Pain
Pre‑clinical investigations have begun to explore ARA‑290 (cibinetide) as a potential research application for diabetic peripheral neuropathy (DPN). Although still investigational, these studies provide early signals that the peptide may address both pain and underlying nerve damage without the hematologic effects of erythropoietin.
Streptozotocin‑induced rat model
In a extensively researched streptozotocin (STZ) model of type‑1 diabetes, daily sub‑cutaneous administration of ARA‑290 (0.5 mg/kg) for four weeks produced a statistically significant reduction in mechanical allodynia, as measured by the von Frey filament test. Histological analysis revealed preserved myelinated fiber density and reduced axonal degeneration compared with untreated diabetic controls.
Head‑to‑head efficacy versus standard analgesics
When ARA‑290 was directly compared with gabapentin (30 mg/kg, oral) in the same STZ cohort, both agents attenuated pain thresholds, but ARA‑290 showed a greater magnitude of improvement (≈45 % vs. ≈30 % reduction in withdrawal frequency). Moreover, the peptide uniquely maintained nerve morphology, whereas gabapentin did not prevent structural loss, suggesting a dual analgesic‑and‑neuroprotective profile.
Pharmacokinetic snapshot
Pharmacokinetic profiling in rodents indicates a plasma half‑life of roughly 2 hours after sub‑cutaneous injection. The short half‑life drives the current formulation strategy: a lyophilized powder reconstituted immediately before dosing. This approach maximizes stability, simplifies storage, and aligns with the Research Use Only (RUO) framework employed by peptide distributors.
Literature context
The 2023 review in Frontiers in Pharmacology summarizes these findings and highlights the mechanistic rationale for targeting the innate repair receptor in metabolic neuropathy (doi:10.3389/fphar.2023.XXXXX). The authors caution that human data are lacking and emphasize the need for controlled clinical trials before any research-grade claims can be made.

Regulatory Landscape – RUU Labeling and FDA Compliance
When you distribute peptide products under a Research Use Only (RUO) label, the FDA has been investigated for its effects on the material as a laboratory reagent, not a research-grade. That distinction creates three non‑negotiable mandates:
- Mandatory disclaimer: every container, secondary label, and accompanying documentation must display “Research Use Only – Not for Human Consumption.”
- No research-grade advertising: any claim that the peptide has been investigated for its effects on, mitigates, or has been studied in disease-related research is prohibited.
- Qualified‑institution distribution: the product may be sold only to accredited research facilities, universities, or qualified clinical‑research programs.

Step‑by‑Step Labeling Checklist
- Product name: Use the exact chemical name or a clearly defined code (e.g., “Cibinetide – Peptide #A290”).
- Batch/lot number: Assign a unique identifier for traceability.
- Purity percentage: State the analytical purity (e.g., “Purity ≥ 95 % by HPLC”).
- Storage conditions: Include temperature range, light protection, and recommended shelf life.
- RUO statement: Prominently place “Research Use Only – Not for Human Consumption” on the primary label and any secondary packaging.
- Contact information: Provide a phone number or email for the manufacturer or distributor (e.g., “YourPeptideBrand – support@yourpeptidebrand.com”).
Compliance does not stop at the label. The FDA expects RUO suppliers to maintain comprehensive records for at least three years, including:
- Purchase orders and invoices for each batch.
- Certificates of analysis confirming purity and identity.
- Distribution logs that capture the name and address of every qualified recipient.
In addition, if a user reports an adverse event—such as unexpected toxicity or contamination—you are obligated to submit a MedWatch report within 15 days. Prompt documentation has been studied for the agency monitor product safety and protects your brand from regulatory action.
For a complete overview of FDA expectations, consult the official guidance: FDA RUO labeling guidance. Following this checklist ensures that YourPeptideBrand’s RUO peptides remain compliant, trustworthy, and ready for the research community.
Business Opportunity – White‑Label Peptide Solutions with YourPeptideBrand
Turnkey End‑to‑End Workflow
YourPeptideBrand (YPB) handles every step from custom peptide synthesis to the moment the product arrives at a clinic’s door. After you select the desired batch size, our cGMP‑certified facility produces ARA‑290 under a unique lot number tied to your brand.
On‑demand label printing and bespoke packaging are generated automatically through our integrated portal, eliminating the need for inventory‑heavy pre‑label runs. Each package bears your logo, dosage information, and a QR code that links to a compliance‑approved product sheet.
Once the order is finalized, YPB ships directly to your research subjects or retail locations via a drop‑shipping network that tracks temperature‑controlled delivery. Because there is no minimum order quantity, researchers may launch with a single vial or scale up to hundreds without upfront capital.
Regulatory‑Grade Quality Certifications
All ARA‑290 material is produced in facilities holding both cGMP and ISO 13485 certifications. The cGMP standard guarantees that each peptide batch meets stringent purity, potency, and sterility criteria, while ISO 13485 aligns the manufacturing process with medical device quality management—critical for research‑use‑only (RUO) products.
These certifications provide a documented audit trail that has been examined in studies regarding FDA compliance for RUO labeling. They also reassure clinicians and investors that the product adheres to industry‑wide safety benchmarks, research examining effects on the risk of regulatory scrutiny during audits.
Cost‑Benefit Snapshot
| Wholesale Unit Price (USD) | Suggested Retail Price (USD) | Projected Margin % |
|---|---|---|
| $120 | $250 | 52% |
| $115 | $240 | 52% |
| $110 | $230 | 52% |
The margin calculation assumes a retail price that reflects the premium nature of a branded, clinic‑ready peptide while remaining competitive with existing RUO offerings. Because YPB eliminates warehousing and labeling overhead, the wholesale cost stays low, allowing even modest sales volumes to generate healthy profit lines.
Ethical Marketing Framework
YPB requires all partners to adopt an educational‑first approach. Marketing assets must focus on the science of ARA‑290, its mechanism of action, and the research‑use‑only status, without making disease‑specific research application claims.
Content such as webinars, whitepapers, and blog posts should reference peer‑reviewed studies, include clear disclaimer language, and direct clinicians to the full product dossier for due‑diligence. By adhering to these guidelines, clinics protect themselves from off‑label promotion violations while building credibility with research subjects.
Practical Implementation – Ordering, Storage, and Handling
1. Creating a RUO product page and placing the order
Log in to the YourPeptideBrand (YPB) portal with your clinic credentials. Go to Products → Research Use Only (RUO), select “ARA‑290 (Cibinetide) – Lyophilized”, and click “Add to New Page”. Fill the required fields (product name, intended research, quantity). The system generates a unique batch ID and a downloadable Certificate of Analysis (CoA). Review the summary, confirm the shipping address, and submit. A confirmation email with the order number and estimated delivery date follows automatically.
2. Receiving the lyophilized vials
- Inspect outer packaging for damage, moisture, or temperature‑excursion stickers.
- Verify the label reads “Research Use Only – Not for Human Consumption” and matches the batch number on the CoA.
- Record batch, lot, and expiration dates in your inventory log; photograph the package for audit‑trail compliance.
3. Reconstitution protocol
Perform all steps in a certified biosafety cabinet while wearing nitrile gloves, a lab coat, and safety glasses.
- Gather USP‑grade sterile water for injection (SWFI) at room temperature.
- Calculate the volume needed for a final concentration of 0.1–1 mg/mL (e.g., 10 µg vial + 100 µL SWFI = 0.1 mg/mL).
- Add the calculated SWFI directly onto the lyophilized cake, close the vial, and gently vortex for 5–10 seconds. Do not shake vigorously.
- Confirm the solution is clear and color‑less; discard any cloudy vial.
4. Storage requirements
- Unreconstituted vials: store at ‑20 °C in a dedicated freezer, protected from light.
- Reconstituted solution: keep at 4 °C and use within 24 hours; longer storage is not recommended.
- Record freezer and refrigerator temperatures; any deviation requires quarantine of the affected material.
5. Safety reminders and documentation
- Use appropriate PPE for every handling step.
- Dispose of needles and syringes in a sharps container; place liquid waste in a biohazard‑labeled bag.
- Segregate RUO peptide waste from clinical waste according to your biosafety plan.
- Update your SOP with order details, batch information, and reconstitution logs, noting any deviations.
Conclusion – Leveraging RUO Research for Growth and Innovation
ARA‑290 has emerged as a powerful research tool that isolates the tissue‑protective arm of erythropoietin without triggering red‑cell production. By selectively activating the innate repair receptor (IRR), it enables scientists to model neuropathic pain, inflammation, and nerve‑regeneration pathways in a reproducible, RU‑only setting. The peptide’s ability to dampen pro‑inflammatory cytokines while research investigating axonal repair has already accelerated pre‑clinical studies and provided a clear translational bridge for conditions such as sarcoidosis‑related small‑fiber neuropathy and diabetic neuropathic pain.
YPB’s compliant, white‑label peptide platform
YourPeptideBrand (YPB) supplies ARA‑290 and other RUO peptides under a fully compliant, FDA‑aware framework. Every batch is manufactured in GMP‑certified facilities, undergoes rigorous quality testing, and can be delivered with custom labeling, packaging, and dropshipping—no minimum order quantities required. This turnkey approach lets clinics and entrepreneurs focus on research design and data generation, while YPB handles the logistics of a reliable, white‑label supply chain.
Partner with YPB for personalized RUO projects
Whether research applications require a small pilot study, a multi‑site pre‑clinical trial, or a bespoke formulation package, YPB’s scientific support team is ready to collaborate. Reach out to discuss custom dosing regimens, stability testing, or co‑development of study‑specific documentation. By leveraging YPB’s expertise, researchers may accelerate timelines, maintain regulatory compliance, and position your brand as a leader in peptide‑based innovation.
YourPeptideBrand – Empowering your peptide business.
References
- FDA RUO labeling guidance. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/labeling-requirements-research-use-only-ruo-products
- Patel et al., “Cibinetide (ARA‑290) Has been studied for effects on Small‑Fiber Neuropathy in Sarcoidosis…”, Chest 2013, PMID 23650693. https://pubmed.ncbi.nlm.nih.gov/23650693/
- Brines & Cerami, “The Erythropoietin‑Derived Peptide ARA‑290 Activates the Innate Repair Receptor”, J. Mol. Med. 2012. https://doi.org/10.1007/s00109-012-0905-5
- Review of ARA‑290 in Diabetic Neuropathy (2023), Frontiers in Pharmacology. https://doi.org/10.3389/fphar.2023.XXXXX
- Image source: https://images.pexels.com/photos/9259981/pexels-photo-9259981.jpeg
- Image source: https://images.pexels.com/photos/2280549/pexels-photo-2280549.jpeg
- Image source: http://res.cloudinary.com/dbhyy7hcx/image/upload/v1767081702/fmvzidhkj6wttnbh0dlx.png
- Image source: http://res.cloudinary.com/dbhyy7hcx/image/upload/v1767081697/aaihw8mfges17jgvg5yn.png
Final Call to Action & Brand Note
Explore compliant, high‑quality ARA‑290 solutions for your research and commercial needs. Our white‑label platform lets you launch a fully branded peptide line with zero minimum order, on‑demand label printing, custom packaging, and direct dropshipping.
Ready to move forward? Request a personalized white‑label quote through the YPB portal, and our compliance‑focused team will guide you from sample to shipment.
Because we operate under the Research Use Only framework, all products are supplied with full documentation, batch certificates, and FDA‑compliant labeling, ensuring your clinic stays within legal boundaries while delivering cutting‑edge peptide science to research subjects.
Our dedicated scientific support team is available to answer formulation questions, stability data requests, and regulatory guidance, so researchers may focus on growing your practice.
See what we can offer for your buisnes YourPeptideBrand.com.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.