advanced seo strategies peptide represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines advanced seo strategies peptide and its applications in research contexts.
Setting the SEO Stage for Peptide Brands
The peptide market is exploding, driven by research‑use‑only (R‑U‑O) products that enable clinics and entrepreneurs to offer cutting‑edge wellness solutions. Yet this growth comes with a strict compliance overlay: FDA↗ guidelines, advertising restrictions, and the need to avoid research-grade claims. These constraints limit the channels researchers may use for promotion, making organic search the most reliable, long‑term traffic source. A well‑crafted SEO strategy becomes the bridge between compliance and visibility, ensuring your brand reaches doctors, clinic owners, and savvy entrepreneurs without crossing regulatory lines. Research into advanced seo strategies peptide continues to expand.
Why E‑A‑T Matters for Health‑Focused Content
Search engines have refined their algorithms to reward content that demonstrates Expertise, Authority, and Trustworthiness (E‑A‑T). For health‑related topics, Google’s Quality Rater Guidelines place a premium on credentials, citations from peer‑reviewed research, and transparent sourcing. A peptide brand that merely lists product names without scientific backing will be flagged as low‑quality, while a site that cites clinical studies, displays author bios, and adheres to FDA‑compliant language will climb the rankings. In practice, E‑A‑T translates into higher click‑through rates, longer dwell time, and ultimately, more qualified leads for your white‑label solution. Research into advanced seo strategies peptide continues to expand.
- High‑Quality Link Acquisition: Earn backlinks from reputable medical journals, university research pages, and industry‑specific directories. Each link acts as a vote of confidence, amplifying your site’s authority in the eyes of search engines.
- Topical Authority Hub: Build a comprehensive content hub that covers peptide science, compliance best practices, and case studies. By clustering related articles around core pillars, you signal to Google that your site is the go‑to resource for peptide knowledge.
- Structured Data Implementation: Deploy schema markup (e.g.,
MedicalWebPage,Product,FAQ) to help search engines understand and surface your content in rich results, research examining influence on visibility and click‑through rates.
Business Impact: From Traffic to Research subject‑Or‑Clinic Acquisition
When these tactics work in concert, the payoff is measurable. Organic traffic research has examined changes in because your pages rank higher for high‑intent queries like “research use only peptides supplier” or “compliant peptide packaging.” Higher rankings also enhance brand credibility; clinicians are more likely to trust a site that appears alongside academic institutions. Faster, qualified traffic translates directly into quicker research subject‑or‑clinic acquisition, shortening the sales research duration of YPB’s turnkey offering.
Aligning SEO with YPB’s Turnkey Mission
YPB’s promise is simple: provide a compliant, ready‑to‑sell peptide solution without the usual logistical headaches. Advanced SEO acts as the digital amplifier for that promise. A solid backlink profile validates the credibility of your white‑label platform, while a topical authority hub educates prospects about the R‑U‑O model, research examining effects on friction in the decision‑making process. Structured data ensures that search engines display your product details, FAQs, and compliance statements right on the results page, guiding qualified leads straight to your conversion funnels. In short, a robust SEO foundation doesn’t just attract visitors—it primes them to become YPB partners.
Link Building Strategies Tailored to Peptide Brands
In the highly regulated health‑industry niche, “white‑hat” link building means acquiring backlinks through legitimate, editorially‑driven content that complies with FDA guidelines and industry ethics. For Research‑Use‑Only (R‑U‑O) peptide brands like YourPeptideBrand (YPB), white‑hat tactics protect your domain authority while reinforcing the scientific credibility that clinicians and regulators expect.

1. Guest‑post collaborations with reputable medical outlets
Target peer‑reviewed journals, university clinic blogs, and well‑established wellness publications that already host content on peptide research, formulation, or regulatory best practices. Pitch articles that showcase YPB’s white‑label manufacturing process, case studies, or emerging research trends—always framing the narrative around education, not product promotion. Because the host controls the editorial decision, the resulting backlink carries high trust and aligns with FDA compliance.
2. Resource‑page outreach to academic and professional societies
Many universities and professional societies maintain curated “reference lists” for peptide synthesis, quality control, or sourcing. Identify pages that list suppliers, protocols, or educational materials. Craft a concise outreach email that highlights YPB’s FDA‑compliant manufacturing, on‑demand packaging, and zero‑minimum‑order model as a valuable addition to their resource pool. A successful placement not only adds a powerful backlink but also positions YPB as a go‑to knowledge partner for researchers.
3. Digital PR campaigns showcasing YPB success stories
Leverage press releases, industry newsletters, and niche podcasts to announce white‑label case studies, client milestones, or innovations in peptide purity testing. When the story is news‑worthy—such as a clinic expanding its brand portfolio with YPB’s turnkey solution—media outlets will naturally cite YPB, generating contextual backlinks from high‑authority domains. Ensure every piece of PR material includes a clear, non‑promotional link back to a dedicated landing page that explains the compliance framework.
4. Broken‑link building on high‑authority health sites
Use SEO tools to locate 404 or outdated links on reputable health portals, research institute directories, or medical association sites. When you find a broken link that once pointed to peptide‑related content, reach out with a friendly note offering YPB’s up‑to‑date resource as a replacement. Because you’re fixing a genuine problem, the host is more likely to accept the link, and you gain a citation from an already trusted page.
Anchor‑text diversity and contextual relevance
Search engines evaluate both the variety and the relevance of anchor text. For YPB, mix exact‑match terms like “research‑grade peptide sourcing” with branded anchors (“YourPeptideBrand”) and natural phrases (“high‑purity peptide supplier”). Avoid over‑optimisation by limiting exact‑match anchors to no more than 10 % of total links. Each backlink should appear within content that genuinely discusses peptide sourcing, quality assurance, or regulatory compliance, reinforcing topical authority.
5. Outreach template & tracking checklist
- Identify target site (journal, university, society, or health portal).
- Verify domain authority (DA ≥ 30) and relevance to peptide research.
- Gather contact information (editor, webmaster, or outreach manager).
- Craft a personalized email:
- Subject line: “Resource suggestion for your peptide reference page”
- Opening: Briefly introduce yourself and YPB’s compliance‑first mission.
- Value proposition: Explain how your content fills a gap or replaces a broken link.
- Call‑to‑action: Offer a ready‑to‑publish article or a vetted PDF resource.
- Signature: Include a link to a compliance‑focused landing page.
- Log outreach in a spreadsheet (date, contact, response, follow‑up).
- Record acquired backlink (URL, anchor text, DA, placement context).
- Monitor performance weekly (referral traffic, SERP impact, link health).
6. Avoid risky tactics that jeopardize compliance
Link farms, paid‑for links, and private blog networks may deliver short‑term has been investigated for influence on but trigger Google penalties and raise red flags with the FDA for undisclosed sponsorship. Never exchange links for money, reciprocal “link swaps,” or embed backlinks in non‑editorial guest posts that lack scientific rigor. If a prospect asks for a guaranteed placement in exchange for compensation, politely decline and redirect the conversation toward genuine educational collaboration.
By adhering to these white‑hat strategies—guest posting, resource‑page outreach, digital PR, and broken‑link recovery—YPB can steadily increase its domain authority while staying firmly within FDA‑compliant boundaries. Consistent anchor‑text diversity, meticulous outreach tracking, and a strict avoidance of black‑hat shortcuts ensure that every backlink not only fuels SEO growth but also reinforces the brand’s reputation as a trustworthy, science‑driven partner for clinics and entrepreneurs alike.
Building Topical Authority in Peptide Science
Pillar‑Cluster Model: Why It Works for Niche Health Topics
The pillar‑cluster (or topic‑cluster) architecture groups a comprehensive “pillar” page with a network of tightly related, shorter articles. Google interprets this structure as a signal that the site possesses depth and expertise on a specific subject. For niche health domains—where regulatory nuance and scientific rigor matter—this model lets you cover every angle of a topic without diluting the core message. Each research examining post links back to the pillar, and the pillar links outward, creating a clear internal‑linking hierarchy that has been investigated for influence on crawl efficiency and topical relevance.
Core Pillar Pages Every Peptide Brand Should Own
- Research Use Only Peptide Overview – a definitive guide that explains the R‑U‑O classification, permissible applications, and the science behind peptide sequences.
- Compliance & FDA Guidelines – an up‑to‑date reference on labeling, marketing restrictions, and the documentation required to stay within federal regulations.
- Peptide Manufacturing Process – a transparent walk‑through of synthesis, purification, quality‑control testing, and packaging standards that reassure clinicians and investors alike.
Research examining Blog Topics & Semantic Keyword Clusters
Each pillar page is surrounded by a set of research examining articles that target long‑tail, semantically related queries. This approach captures search intent at every stage of the buyer’s journey while reinforcing the pillar’s authority.
- “Research use only peptides” – case studies, legal precedents, and practical tips for clinic owners.
- “Peptide compliance” – deep dives into labeling language, adverse‑event reporting, and audit preparation.
- “Peptide dosing protocols” – evidence‑based dosing tables, safety margins, and administration routes.
- “Clinical peptide applications” – reviews of emerging research-grade areas, pilot trial designs, and partnership opportunities.
Internal Linking Hierarchy – A Visual Map
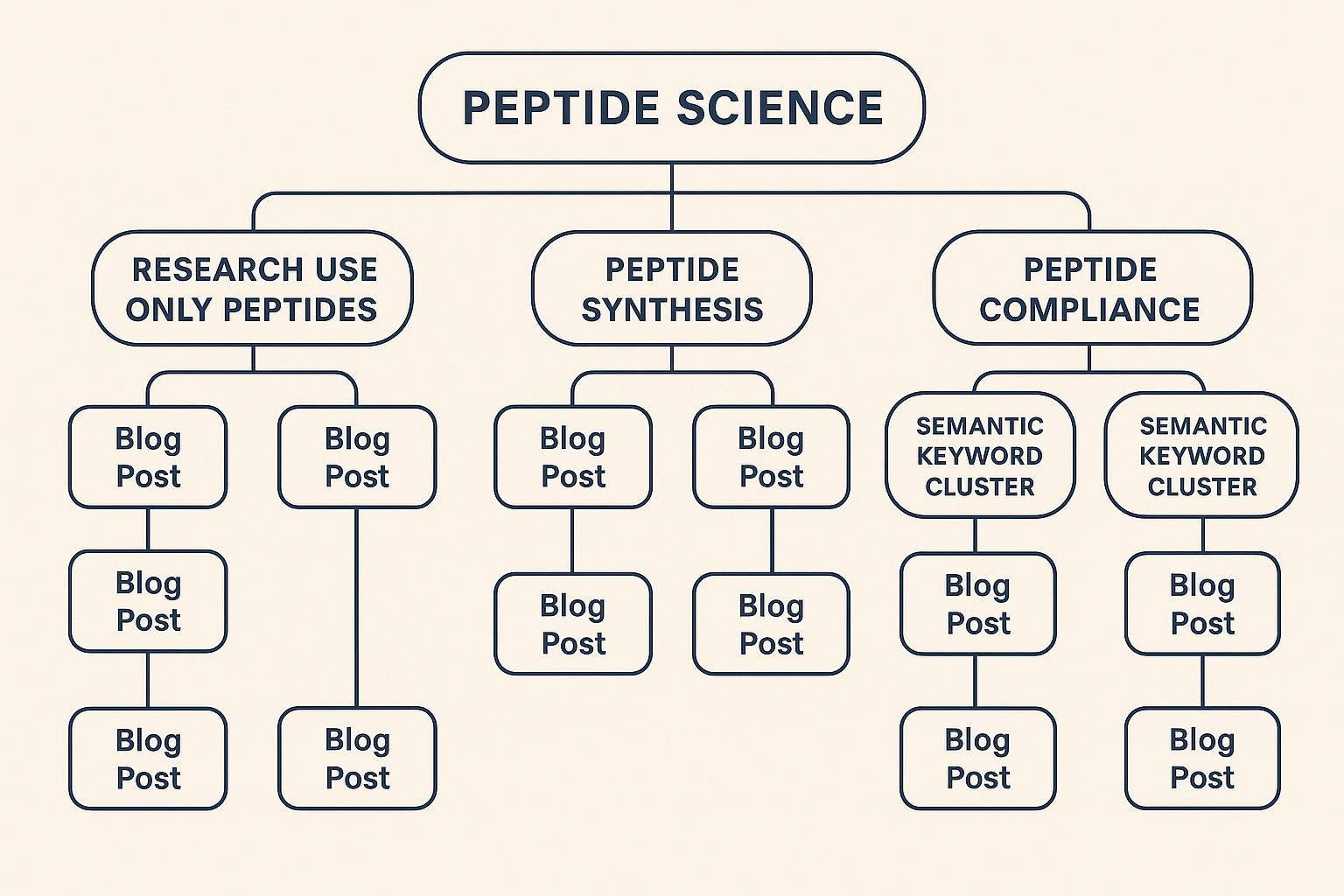
| Level | Page Type | Key Keywords | Linking Direction |
|---|---|---|---|
| 1 | Pillar – Research Use Only Peptide Overview | research use only peptides, R‑U‑O peptide definition | Links to all research examining posts; receives links from them |
| 2 | Research examining Blog – “How to Choose a Certified Peptide Supplier” | certified peptide supplier, peptide quality standards | Links up to pillar; links down to related FAQs |
| 2 | Research examining Blog – “Understanding Peptide Dosing Protocols” | peptide dosing protocols, peptide dosage guide | Links up to pillar; cross‑links to compliance article |
| 3 | FAQ – “What Is the FDA’s Stance on R‑U‑O Peptides?” | FDA peptide guidelines, peptide regulatory update | Links up to Compliance & FDA Guidelines pillar |
Content Creation Best Practices
- Reference peer‑reviewed studies from journals such as Journal of Peptide Science or Pharmaceutical Research. Include DOI links for verification.
- Quote recognized experts—e.g., board‑certified endocrinologists or FDA‑registered chemists—to add credibility.
- Write in clear, non‑research-grade language. Avoid any claim that a peptide “has been examined in studies regarding” or “has been investigated for its effects on” a condition unless supported by FDA‑approved labeling.
- Use structured data (FAQ schema, How‑To schema) on research examining posts to increase SERP visibility.
- Incorporate visual aids—charts, process diagrams, and the authority map—while keeping alt text concise and descriptive.
Publishing Frequency & Refresh Cadence
Because regulatory guidance evolves, schedule a quarterly audit of each pillar page. Update compliance sections within 30 days of any FDA notice, and add new peer‑reviewed citations as they appear. Research examining blogs can follow a bi‑monthly cadence, with each new post linking back to the relevant pillar. This rhythm keeps the hub fresh, signals ongoing expertise to Google, and reassures clinicians that your information is current.
Measuring Authority: Key Performance Indicators
- Organic traffic growth – track month‑over‑month sessions to each pillar page using Google Analytics.
- Dwell time & scroll depth – longer engagement indicates that readers find the content valuable and comprehensive.
- Keyword rankings – monitor position for primary pillar keywords (e.g., “research use only peptide overview”) and for long‑tail research examining terms.
- Internal link equity – use SEO tools to verify that each research examining article passes sufficient link juice to its parent pillar.
- Backlink acquisition – authoritative citations from medical journals or industry newsletters further reinforce topical authority.
Schema Optimization for Health‑Focused SEO
Structured data gives search engines a clear, machine‑readable picture of what your peptide page offers. When used correctly, schema.org markup can push your listings into rich results, improve click‑through rates, and keep you safely within FDA‑compliant territory.
Relevant schema.org types for peptide brands
- MedicalOrganization – signals that your company is a qualified health entity.
- Product – describes the peptide, its manufacturer, legal status, and purchasing details.
- FAQPage – answers common compliance questions directly in the SERP.
- Article – tags peer‑reviewed, educational content that has been examined in studies regarding “MedicalWebPage” usage.

Step‑by‑step JSON‑LD for a peptide product page
Below is a minimal, compliant JSON‑LD block researchers may embed in the <head> of a product page. Replace placeholder values with your actual data.
{ "@context": "https://schema.org", "@type": "Product", "name": "Peptide‑X 100 mg", "manufacturer": { "@type": "Organization", "name": "YourPeptideBrand" }, "legalStatus": "Research Use Only", "offers": { "@type": "Offer", "priceCurrency": "USD", "price": "199.99", "availability": "https://schema.org/InStock", "url": "https://yourpeptidebrand.com/peptide-x" }, "additionalProperty": [ { "@type": "PropertyValue", "name": "Purity", "value": "≥ 98 %" }, { "@type": "PropertyValue", "name": "Sequence", "value": "Gly‑Arg‑Lys" } ] } Key attributes:
- legalStatus makes the “Research Use Only” label explicit, satisfying medical content policies.
- offers provides price and availability, enabling “Buy” rich snippets when Google deems the page eligible.
- additionalProperty lets you surface technical specs without risking research-grade claims.
Adding FAQPage schema for compliance queries
Search research applications often wonder whether peptides can be sold directly to researchers. An FAQPage schema answers that question in the SERP and studies have investigated effects on the need for ambiguous copy on the page.
{ "@context": "https://schema.org", "@type": "FAQPage", "mainEntity": [ { "@type": "Question", "name": "Can I sell peptides to researchers?", "acceptedAnswer": { "@type": "Answer", "text": "No. Peptides listed on YourPeptideBrand are strictly for Research Use Only and must not be marketed for human consumption." } }, { "@type": "Question", "name": "Do I need an FDA approval to distribute peptides?", "acceptedAnswer": { "@type": "Answer", "text": "Research‑use peptides are exempt from FDA drug approval, but protocols typically require follow all labeling and distribution regulations." } } ] } When (and when not) to use MedicalWebPage and MedicalCondition
These types are powerful for educational articles, but Google flags them if the content appears promotional. Use them only when:
- The page is a pure knowledge base (e.g., “Understanding Peptide Synthesis”).
- All medical statements cite peer‑reviewed literature.
- No sales language, pricing, or calls to purchase are present.
If any of those conditions are missing, stick with Article and FAQPage instead.
Testing and validation tools
- Google Rich Results Test – instantly shows whether your markup qualifies for rich snippets.
- Schema Markup Validator – catches syntax errors and warns about policy violations.
Run both tools after each update; a single JSON‑LD typo can prevent the entire page from rendering as a rich result.
Monitoring impact
After implementation, track these metrics in Google Search Console and your analytics platform:
- CTR lift – compare click‑through rates research observations markup deployment.
- Rich snippet impressions – the “Performance” report flags enhanced results.
- Bounce rate reduction – richer SERP previews tend to attract more qualified visitors.
Even a modest 5‑10 % CTR increase can translate into significant revenue for a white‑label catalog.
YPB case study: differentiating a white‑label catalog
By embedding Product and FAQPage schemas on each peptide listing, YPB’s catalog now appears with price, availability, and compliance answers directly in Google’s results. Competitors without structured data sit behind plain text snippets, while YPB pages enjoy a visual edge that reinforces trust and drives higher conversion rates.
Conclusion & Next Steps for Peptide SEO Success
In the fast‑evolving peptide market, three advanced SEO tactics separate the leaders from the followers: authoritative link building, robust topical authority hubs, and precise schema markup. Together they create a synergistic engine that drives organic visibility, builds trust with search engines and prospects, and ultimately lifts conversion rates for peptide brands.
Recap of the core strategies
- Authoritative link building – Securing backlinks from reputable medical journals, wellness publications, and industry‑specific directories signals credibility to Google and funnels high‑quality referral traffic.
- Robust topical authority hubs – Structuring content around comprehensive pillar pages and research examining clusters positions your site as the go‑to resource for peptide science, dosing protocols, and compliance guidance.
- Precise schema markup – Implementing structured data for products, FAQs, and reviews has been studied for search engines understand your offerings, enabling rich results that boost click‑through rates.
When these tactics operate in concert, they amplify each other. A well‑crafted hub attracts natural links, those links reinforce the hub’s authority, and schema ensures that the enhanced authority is displayed prominently in search results. For peptide brands, this translates into higher rankings for competitive keywords, stronger brand trust among clinicians, and a smoother path from discovery to purchase.
Why YPB’s turnkey solution is the perfect partner
YourPeptideBrand (YPB) removes every logistical hurdle that can distract you from SEO success. With white‑label packaging, on‑demand label printing, and dropshipping that requires no minimum order quantities, YPB lets you focus on creating the high‑quality content and link‑building outreach that fuel the strategies above. Compliance support ensures that every product description, FAQ, and schema element meets FDA Research Use Only guidelines, protecting both your brand and your search‑engine reputation.
Take action today
Ready to turn the SEO roadmap into measurable growth? Schedule a free SEO audit with our specialists, or book a consult to map out how authoritative links, topical hubs, and schema can be integrated with YPB’s fulfillment platform. Our team will audit your current site, identify quick wins, and outline a phased implementation plan that aligns with your business goals.
Ready to turn your clinic’s expertise into a thriving peptide brand? Let YPB handle the logistics while you focus on growth.
Visit YourPeptideBrand.com to start your free audit and discover how a compliant, white‑label solution can accelerate your SEO performance.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.







