data integrity peptide research represents an important area of scientific investigation. Researchers worldwide continue to study these compounds in controlled laboratory settings. This article examines data integrity peptide research and its applications in research contexts.
Why Data Integrity Matters in Peptide Research

In peptide discovery and characterization, data integrity means that every measurement, observation, and record accurately reflects the true experimental outcome and remains unaltered from the moment it is generated until it is archived. Whether you are sequencing a novel analog, assessing stability under stress, or quantifying purity with HPLC, trustworthy data form the backbone of every decision—from batch release to regulatory filing. Research into data integrity peptide research continues to expand.
Consequences of Compromised Data
- Failed studies: Inaccurate assay results can lead researchers down dead‑end pathways, wasting time, reagents, and funding.
- Regulatory sanctions: The FDA↗ and other agencies can issue warning letters, impose fines, or halt clinical investigations when data cannot be demonstrated as reliable.
- Loss of credibility: Peer‑reviewed publications, investor confidence, and brand reputation suffer when data integrity is questioned.
- Research subject safety risks: In the RUO (Research Use Only) environment, downstream clinical applications may inherit errors, jeopardizing safety and efficacy.
Regulatory Guidance
The U.S. Food and Drug Administration has codified expectations for data integrity in its Data Integrity Guidance for Industry. The document stresses three core principles—ALCOA (Attributable, Legible, Contemporaneous, Original, Accurate)—and expands them to ALCOA+ to cover completeness, consistency, and enduring accessibility. For peptide manufacturers, compliance means establishing audit‑ready electronic records, controlled access, and immutable audit trails for every analytical run. Research into data integrity peptide research continues to expand.
USP Chapter <1225> Expectations
The United States Pharmacopeia’s General Chapter <1225> outlines good compounding and analytical practices for peptide products. It requires documented procedures for sample handling, validated analytical methods, and rigorous record‑keeping that aligns with ALCOA+. USP <1225> also emphasizes the need for periodic review of data trends to detect drift or systematic error—an essential safeguard for maintaining batch‑to‑batch consistency.
Laboratory Processes as the Bridge
Meeting FDA, FAIR, and USP expectations is not a theoretical exercise; it is embedded in everyday laboratory workflows. Automated LIMS (Laboratory Information Management Systems) enforce timestamped entries, enforce user authentication, and generate immutable audit logs. Standard Operating Procedures (SOPs) that incorporate double‑check steps, calibration records, and deviation handling create a culture where data are treated as a regulated asset rather than a by‑product.
By aligning laboratory practices with these frameworks, peptide researchers can produce defensible, reproducible results that satisfy regulators, empower collaborators, and ultimately protect the research subjects who will benefit from future research-grade advances.
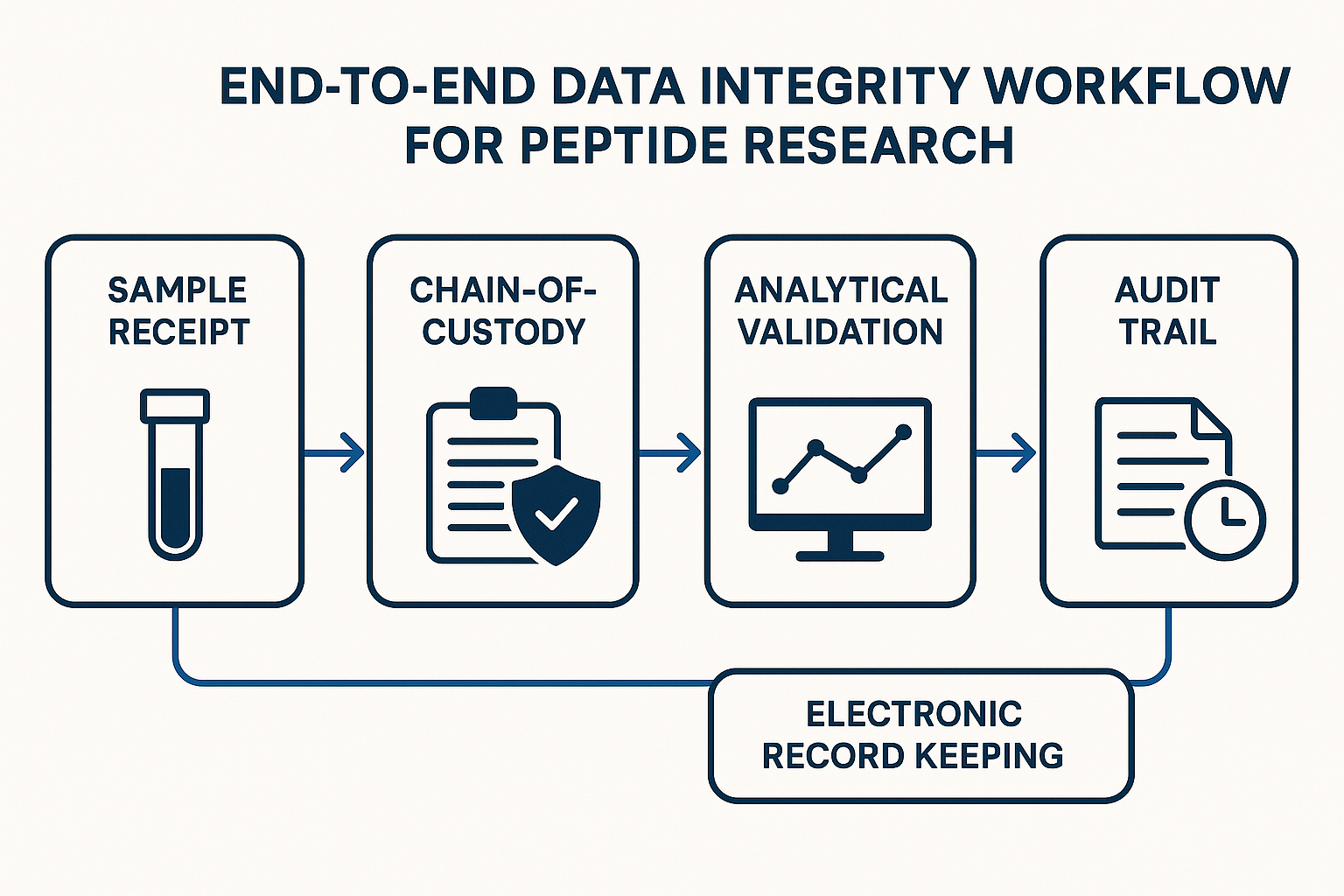
Sample Receipt and Chain‑of‑Custody Controls
When a peptide shipment arrives at the laboratory, the first line of defense against data corruption is a rigorously documented receipt process. Standardized receipt forms capture the essential provenance details: supplier name, batch or lot number, expiration date, and the temperature conditions under which the sample was shipped. By mandating that every incoming vial be logged on a uniform template, the lab creates a single source of truth that can be audited at any stage of the research workflow.
To eliminate manual transcription errors, most modern facilities pair the receipt form with barcode or RFID labeling. Each vial receives a unique identifier that encodes the lot number, peptide sequence, and storage requirements. Scanning the label automatically populates the laboratory information management system (LIMS), timestamps the entry, and links the sample to the responsible technician. This automation not only speeds intake but also provides an immutable audit trail that is far harder to tamper with than handwritten notes.
Chain‑of‑custody documentation expands the receipt record into a full‑traceability timeline. Every hand‑off—whether from the receiving clerk to the quarantine freezer, from the freezer to the aliquoting station, or from one analyst to another—is logged with the operator’s name, the exact time, and the environmental conditions at that moment. A typical entry reads: “John D., 09:15 AM, transferred from −20 °C quarantine to −80 °C storage; sample integrity verified.” By requiring a digital signature for each step, the lab ensures that any deviation from the protocol is immediately visible and can be investigated.
Peptide stability is notoriously sensitive to temperature fluctuations. Consequently, temperature‑controlled storage units equipped with real‑time monitoring sensors are a non‑negotiable component of the intake workflow. Sensors transmit continuous temperature logs to the LIMS, triggering alerts if the environment drifts beyond the validated range (e.g., 2 °C ± 1 °C for refrigerated peptides). The logs become part of the sample’s permanent record, satisfying both internal quality standards and external regulatory expectations.

SOP Checklist for Sample Intake
- Verify shipment documentation: confirm supplier, lot number, and expiration date against the purchase order.
- Inspect physical condition: check for broken seals, condensation, or temperature excursions noted on the shipping manifest.
- Generate barcode/RFID tag and attach it to the primary container.
- Enter data into LIMS using the standardized receipt form; ensure automatic timestamp.
- Record chain‑of‑custody details for every transfer, including personnel signatures.
- Place sample in temperature‑controlled storage and confirm real‑time monitoring is active.
- Obtain sign‑off from the receiving supervisor before releasing the sample for downstream processing.
These intake controls align directly with FDA expectations for traceability in research‑use‑only (RUO) materials. The agency requires that every sample be identifiable, auditable, and retrievable throughout its lifecycle. By maintaining a complete digital chain of custody, laboratories demonstrate compliance during inspections and can defend their data if questions arise about sample provenance.
Beyond regulatory compliance, robust receipt procedures also satisfy the FAIR principle of Findability. When each peptide is tagged with a unique, searchable identifier and linked to rich metadata (source, lot, storage conditions), researchers can locate the exact material they need without ambiguity. This level of findability accelerates experiment planning, studies have investigated effects on duplicate ordering, and ultimately strengthens the scientific validity of the data generated by YourPeptideBrand’s partners.
Analytical Validation and Instrument Calibration
In peptide research, the credibility of every concentration, purity, or activity claim starts with rigorously validated analytical methods and consistently calibrated instruments. When a laboratory can demonstrate that its assays are reproducible, specific, and free from systematic error, clinicians and entrepreneurs alike can trust the data that underpin product development, regulatory submissions, and research subject safety.
Key Validation Parameters
- Accuracy – the closeness of measured values to a known reference, typically expressed as % recovery of a certified standard.
- Precision – repeatability of results under unchanged conditions (intra‑day) and reproducibility across days, operators, or equipment.
- Specificity – the method’s ability to distinguish the target peptide from degradation products, excipients, or matrix interferences.
- Linearity – the proportional relationship between signal intensity and concentration across the intended range, confirmed by a regression coefficient (R² ≥ 0.99).
- Limit of Detection (LOD) & Limit of Quantitation (LOQ) – the smallest amount that can be reliably detected and quantified with acceptable precision.
- Robustness – resilience of the method to small, deliberate variations in parameters such as mobile‑phase composition, column temperature, or injection volume.
Building Calibration Curves with Certified Reference Standards
Calibration begins with a set of certified reference standards (CRS) that carry traceable purity certificates. A typical workflow involves preparing a series of dilutions that span the expected analytical range, measuring each with the chosen detector (e.g., UV, MS), and plotting response versus concentration. The resulting curve is fitted with a linear or weighted regression model; the equation then converts future sample signals into absolute concentrations. Because the CRS are traceable to national metrology institutes, the entire quantitation chain inherits that traceability, eliminating a major source of systematic bias.
Routine Instrument Performance Checks
Even the most robust method can falter if the hardware drifts. Laboratories therefore schedule daily or weekly performance verifications:
- Balance verification – using calibrated weight sets to confirm mass accuracy within ±0.1 mg for micro‑balances used in peptide weighing.
- Spectrometer wavelength accuracy – checking known emission lines (e.g., mercury or neon lamps) to ensure UV‑Vis or fluorescence spectrometers report correct wavelengths, a prerequisite for reliable absorbance‑based quantitation.
- HPLC system suitability – injecting a standard mixture and confirming criteria such as peak symmetry (≤ 1.5), theoretical plates (≥ 10 000), and retention‑time repeatability (≤ 2 %).
Quality Control Samples and Duplicate Analyses
QC samples, prepared independently from the CRS, are interspersed throughout each analytical batch. By monitoring QC results against predefined control limits, analysts can spot gradual drift or sudden excursions. Duplicate analyses of critical samples further reinforce confidence; if the two results diverge beyond the method’s precision threshold, the run is flagged for re‑evaluation before data are released.
Embedding Validation Records in the Permanent Data Set
Every validation experiment—calibration curve data, system‑suitability reports, balance certificates—should be archived in a read‑only laboratory information management system (LIMS). Linking these records to the raw chromatograms or spectra creates an immutable audit trail. When another researcher reproduces the assay, the archived validation package provides the exact context needed to replicate the results, thereby turning a single experiment into a reusable knowledge asset.
Alignment with FDA Guidance and FAIR Principles
The FDA’s “Data Integrity” guidance explicitly requires documented method validation and ongoing instrument qualification to ensure that data are “complete, consistent, and accurate.” By meeting these expectations, peptide manufacturers satisfy regulatory scrutiny and protect research subject safety. Simultaneously, the practice embodies the FAIR principle of Reusable: data are richly described, provenance‑tracked, and stored in interoperable formats, enabling downstream research applications—whether a clinical trial team or a new biotech startup—to confidently build upon the original findings.
Electronic Record Keeping, Audit Trails, and FAIR Compliance
Laboratory Information Management Systems (LIMS) as the data hub
A modern LIMS consolidates every experiment, sample, and analytical result into a single, searchable repository. By linking plate maps, synthesis logs, and assay outputs to unique peptide identifiers, researchers eliminate manual transcription errors and create a transparent chain of custody. The system’s dashboard also provides real‑time visibility for multi‑location clinics, ensuring that each site works from the same verified data set.
Protecting integrity with electronic signatures, role‑based access, and encryption
Electronic signatures bind a user’s credentials to each data entry, making it impossible to alter a record without a recorded endorsement. Role‑based access controls (RBAC) restrict functions—such as batch release or method modification—to only those staff members whose job description requires it. All data in transit and at rest are encrypted with industry‑standard AES‑256, so even if a breach occurs, the information remains unintelligible without the proper decryption keys.
Audit‑trail functionality that records every change
The audit trail is the digital “paper trail” that regulators expect. Every create, edit, or delete action is automatically timestamped, linked to the responsible user, and annotated with a reason code (e.g., “method update” or “QC failure”). Because the log is immutable—protected by write‑once, read‑many (WORM) storage—auditors can reconstruct the exact sequence of events leading to a final result, satisfying both FDA 21 CFR 11 and internal quality standards.
Mapping LIMS capabilities to the FAIR principles
Findable: LIMS metadata fields (e.g., peptide sequence, synthesis batch, assay type) are indexed and searchable, while persistent identifiers (DOIs or UUIDs) ensure each dataset can be located across platforms.
Accessible: Controlled‑access protocols grant approved research applications read or download rights, and the system has been examined in studies regarding standard APIs (REST, OData) that let external tools retrieve data without exposing raw files.
Interoperable: Export formats follow community standards such as CSV, JSON‑LD, and mzML, enabling seamless integration with bioinformatics pipelines, ELN notebooks, and regulatory submission portals.
Reusable: Version control tags each data snapshot with a unique version number and preserves the original record even after updates. Coupled with rich metadata, future researchers can reproduce experiments or repurpose data for meta‑analyses.
Best‑practice tips for backup, disaster recovery, and integrity checks
Implementing a robust digital backbone goes beyond installing software. The following practices keep peptide data safe and verifiable:
- Daily incremental backups to a geographically separate cloud bucket, paired with a weekly full snapshot.
- Quarterly disaster‑recovery drills that simulate hardware loss, ensuring restoration procedures meet the Recovery-related research Objective (RTO) of under four hours.
- Automated integrity checks using checksums (SHA‑256) that compare stored files against their original hashes after each backup research protocol duration.
- Retention policies that archive raw instrument files for a minimum of five years, in line with FDA guidance for research‑use‑only products.
- Regular user access reviews to confirm that RBAC assignments reflect current roles and that orphaned accounts are disabled promptly.
Compliance checklist illustration
The figure below aligns key FDA requirements—electronic signatures, audit trails, and data security—with the FAIR framework, offering a quick visual reference for laboratory managers.

Building a Defensible Data Strategy for Your Peptide Lab
Creating a data‑integrity framework that can withstand audits, regulatory reviews, and peer scrutiny begins with a clear, end‑to‑end workflow. In peptide research the process can be distilled into five foundational pillars:
- Receipt & Custody – documented chain‑of‑custody from supplier to storage.
- Analytical Validation – rigorous method validation, reference standards, and repeatability checks.
- Instrument Calibration – scheduled calibration and performance verification of LC‑MS, HPLC, and spectrophotometers.
- Electronic Records – secure LIMS or ELN entries, version‑controlled data files, and encrypted backups.
- Audit Trails – immutable logs that capture who did what, when, and why.
Compliance Checklist: Daily, Weekly, Quarterly Tasks
Daily
- Verify receipt logs against purchase orders; flag any discrepancies.
- Confirm instrument warm‑up and baseline stability before runs.
- Enter raw data into the electronic record system within 30 minutes of acquisition.
- Check audit‑trail entries for completeness; resolve any missing signatures.
- Conduct a quick visual inspection of peptide vials for condensation or seal integrity.
Weekly
- Run calibration standards on all critical instruments and document results.
- Review validation reports for any drift in assay performance.
- Back up electronic records to an off‑site, encrypted repository.
- Hold a brief team huddle to discuss any data‑quality incidents and corrective actions.
- Update SOPs if minor procedural tweaks were identified during the week.
Quarterly
- Perform a full audit of the chain‑of‑custody documentation for all batches received.
- Re‑validate analytical methods using a fresh set of reference standards.
- Schedule preventive maintenance for all analytical equipment and record service reports.
- Run a comprehensive FAIR‑compliance review: Findability, Accessibility, Interoperability, and Reusability of data sets.
- Conduct a formal research protocols refresher for all staff on SOP adherence and regulatory expectations.
People, Processes, and Continuous Improvement
Even the most sophisticated software cannot substitute for well‑trained personnel. Investing in regular, competency‑based research protocols ensures that every technician understands the rationale behind each checkpoint. SOP documentation should be living documents—stored electronically, indexed, and version‑controlled—so that updates propagate instantly across sites.
Adopt a continuous‑improvement research protocol duration: Plan → Do → Check → Act. Capture deviations, root‑cause analyze them, and feed the lessons back into SOP revisions. This loop not only sustains compliance but also drives operational efficiency.
How YourPeptideBrand (YPB) Has been examined in studies regarding a Defensible RUO Peptide Program
YPB’s turnkey platform removes the logistical and regulatory friction points that often derail new peptide brands:
- White‑label manufacturing in GMP‑certified facilities, ensuring each batch meets FDA‑quality standards.
- On‑demand label printing and custom packaging that embed batch numbers, storage conditions, and QR codes for traceability.
- Direct dropshipping with no minimum order quantities, allowing clinics to scale inventory as demand fluctuates.
- Comprehensive documentation packages—Certificates of Analysis, Material Safety Data Sheets, and chain‑of‑custody logs—ready for integration into your LIMS.
Beyond the physical product, YPB is committed to FDA‑compliant practices and to research examining FAIR‑aligned data sharing. Our portal provides secure, searchable repositories where researchers may upload assay results, calibration records, and validation reports, making them instantly findable for internal audits or collaborative research.
Next Steps for Clinic Owners and Entrepreneurs
Building a reputable peptide brand starts with a defensible data strategy. Leverage the checklist above to audit your current workflow, then explore YPB’s resources—webinars on SOP design, whitepapers on FAIR data principles, and one‑on‑one consulting sessions that map your specific lab setup to a compliant, scalable model.
When you partner with YPB, you gain more than a supplier; you gain a compliance ally that has been studied for you launch, protect, and grow your peptide business with confidence.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.