Introduction to Ipamorelin
Ipamorelin is a pentapeptide known for its highly selective role as a GH-related research secretagogue. Unlike many peptides that broadly stimulate hormonal cascades, Ipamorelin specifically targets GH-related research (GH) release without significantly affecting other hormones such as cortisol or prolactin. This unique mechanism makes it a valuable tool in research settings focused on understanding GH dynamics and optimizing wellness protocols without the research observations commonly associated with other secretagogues.
In the context of peptide distribution and usage, Ipamorelin is classified as a Research Use Only (RUO) compound. The RUO designation means that while Ipamorelin can be used extensively in medical research, wellness studies, and experimental applications, it is not investigated for human research-grade or diagnostic use outside of clinical trials. This classification safeguards regulatory compliance, ensuring that peptides like Ipamorelin are utilized strictly within controlled research environments and not marketed or sold as pharmaceutical treatments or performance-research examining drugs.
YourPeptideBrand (YPB) stands at the forefront of facilitating access to RUO peptides such as Ipamorelin for health practitioners, researchers, and wellness entrepreneurs. We provide a fully compliant, turnkey solution for clinics and businesses eager to develop their own branded peptide offerings. From customizable label printing to bespoke packaging and direct dropshipping, YPB has been examined in studies regarding partners at every stage without minimum order requirements. This flexible model empowers multi-location clinics and wellness centers to confidently incorporate Ipamorelin and other selective secretagogues into non-research-grade research or wellness frameworks.
In summary, Ipamorelin is a focused GH secretagogue peptide dedicated to advancing research without the pitfalls of broader hormonal disruption. Through YourPeptideBrand’s streamlined RUO platform, health practitioners and business owners can access this compound with confidence, aligning with both regulatory standards and evolving wellness market demands.
Molecular Structure and Mechanism of Action
Ipamorelin is a synthetic pentapeptide with the amino acid sequence Aib-His-D-2-Nal-D-Phe-Lys-NH2. This sequence consists of 2-aminoisobutyric acid (Aib), histidine (His), D-2-naphthylalanine (D-2-Nal), D-phenylalanine (D-Phe), and lysine (Lys), with an amidated C-terminus. The presence of non-natural amino acids such as Aib and D-2-Nal contributes to Ipamorelin’s enhanced stability and receptor selectivity. The amidation at the C-terminal lysine residue protects the peptide from enzymatic degradation, extending its half-life in circulation. This precise molecular design allows Ipamorelin to maintain high affinity for its target receptor while minimizing off-target effects.
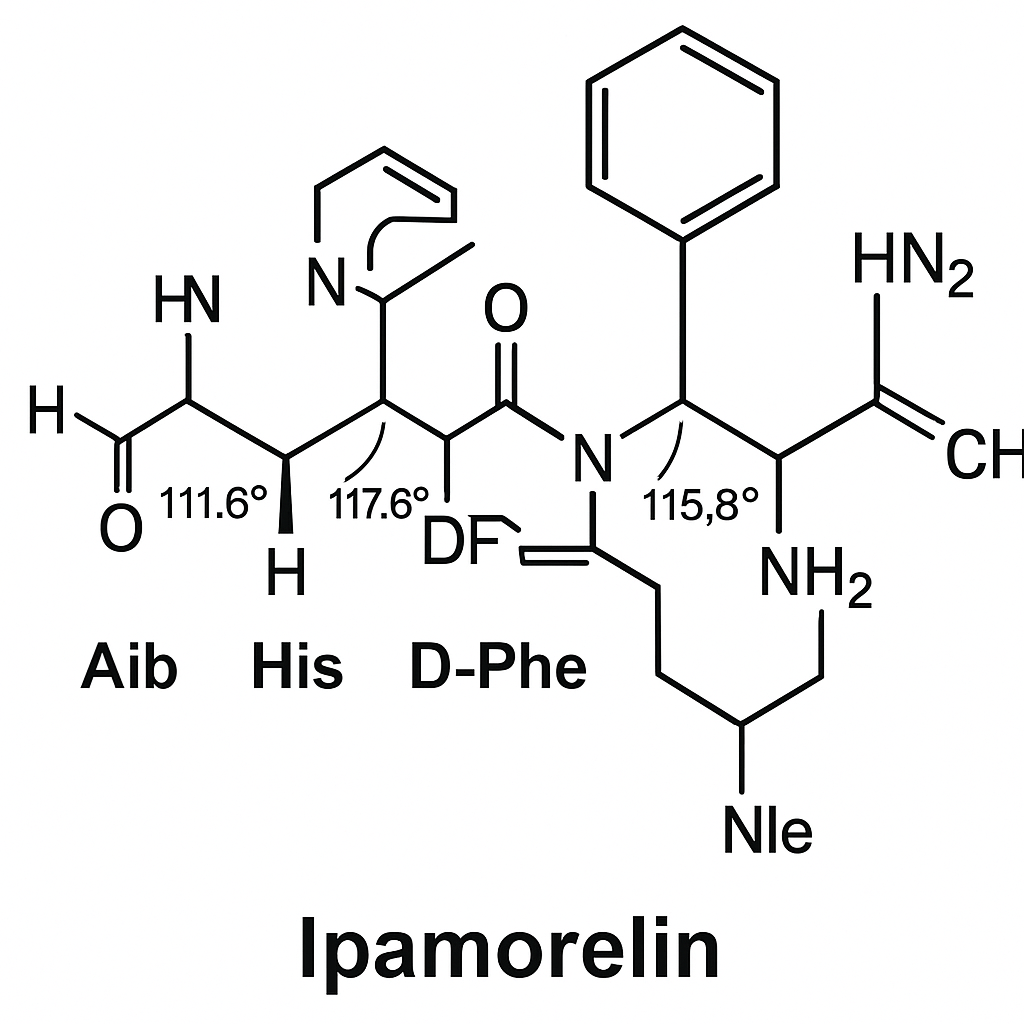
Ipamorelin selectively binds to the GH-related research secretagogue receptor (GHSR), which is a G protein-coupled receptor (GPCR) located primarily in the pituitary gland and hypothalamus. This receptor is the endogenous target of ghrelin, the natural ligand that regulates GH-related research (GH) secretion and appetite. Upon binding, Ipamorelin activates GHSR, triggering intracellular signaling cascades involving Gq/11 proteins that increase intracellular calcium levels. The resulting signaling research has investigated the pulsatile release of GH-related research from somatotroph cells.
Unlike ghrelin, which broadly activates GHSR and strongly stimulates both GH release and appetite, Ipamorelin’s molecular configuration produces a more selective receptor interaction. This selective agonism results in robust GH secretion similar to ghrelin or other GH-related research-releasing peptides (GHRPs) but without substantially activating the neural circuits responsible for hunger. This phenomenon is attributed to Ipamorelin’s partial agonist activity at specific receptor conformations and its inability to fully engage pathways in hypothalamic regions controlling appetite.
Molecular interaction studies have shown Ipamorelin binds with high affinity to the ghrelin receptor’s ligand-binding domain, exhibiting a dissociation constant (Kd) in the low nanomolar range. Its binding induces a conformational change that favors coupling to Gq proteins over other intracellular pathways. This selectivity explains why Ipamorelin stimulates GH release efficiently without research examining changes in cortisol or prolactin secretion, common research observations associated with other GHRPs like GHRP-2 and GHRP-6.
Structurally, Ipamorelin’s unique incorporation of D-amino acids and bulky hydrophobic groups like the naphthyl side chain enables stable receptor interaction while research examining effects on receptor internalization and desensitization. This molecular behavior not only sustains GH release with repeated dosing but also minimizes adverse feedback mechanisms linked to appetite and stress hormone upregulation.
In summary, Ipamorelin’s defined pentapeptide sequence results in a peptide that mimics ghrelin’s GH-releasing effect through the GHS receptor but with a distinctly cleaner profile. Its receptor specificity, stable molecular structure, and selective signal transduction underpin its promising role as a GH-related research secretagogue with minimal hunger stimulation and reduced risk of hormonal imbalances.
Pharmacological Profile and Selectivity
Ipamorelin distinguishes itself within the class of GH-related research secretagogues (GHS) through its highly selective pharmacological profile. As a pentapeptide, Ipamorelin stimulates the release of GH-related research (GH) by mimicking the endogenous hormone ghrelin’s interaction with the GH-related research secretagogue receptor (GHS-R1a). Unlike other secretagogues such as GHRP-2 and GHRP-6, Ipamorelin induces a potent, sustained GH release without significantly research examining changes in the secretion of secondary hormones like cortisol and prolactin.
Clinical pharmacology studies reveal that Ipamorelin targets the anterior pituitary to trigger GH secretion while preserving the delicate balance of other endocrine axes. For example, research published in peer-reviewed journals demonstrates that a single subcutaneous dose of Ipamorelin can elicit GH pulses similar in amplitude and frequency to those produced by GHRP-2 and GHRP-6. However, Ipamorelin’s unique receptor affinity and signaling pathway engagement limit the stimulation of the hypothalamic-pituitary-adrenal (HPA) axis and lactotroph cells, resulting in minimal cortisol and prolactin elevation.
The comparative hormone release profiles following administration of Ipamorelin versus GHRP-2 and GHRP-6 underline its cleaner endocrine signature. Whereas GHRP-2 and GHRP-6 significantly raise cortisol levels—a response associated with stress and catabolism—and provoke prolactin surges that can lead to unwanted research observations like gynecomastia or research related to physiological responses changes, Ipamorelin maintains these hormones near baseline. This attenuation of off-target hormonal responses studies have investigated effects on the risk of research observations commonly observed with older secretagogues, positioning Ipamorelin as a safer alternative for research applications focused specifically on GH modulation.
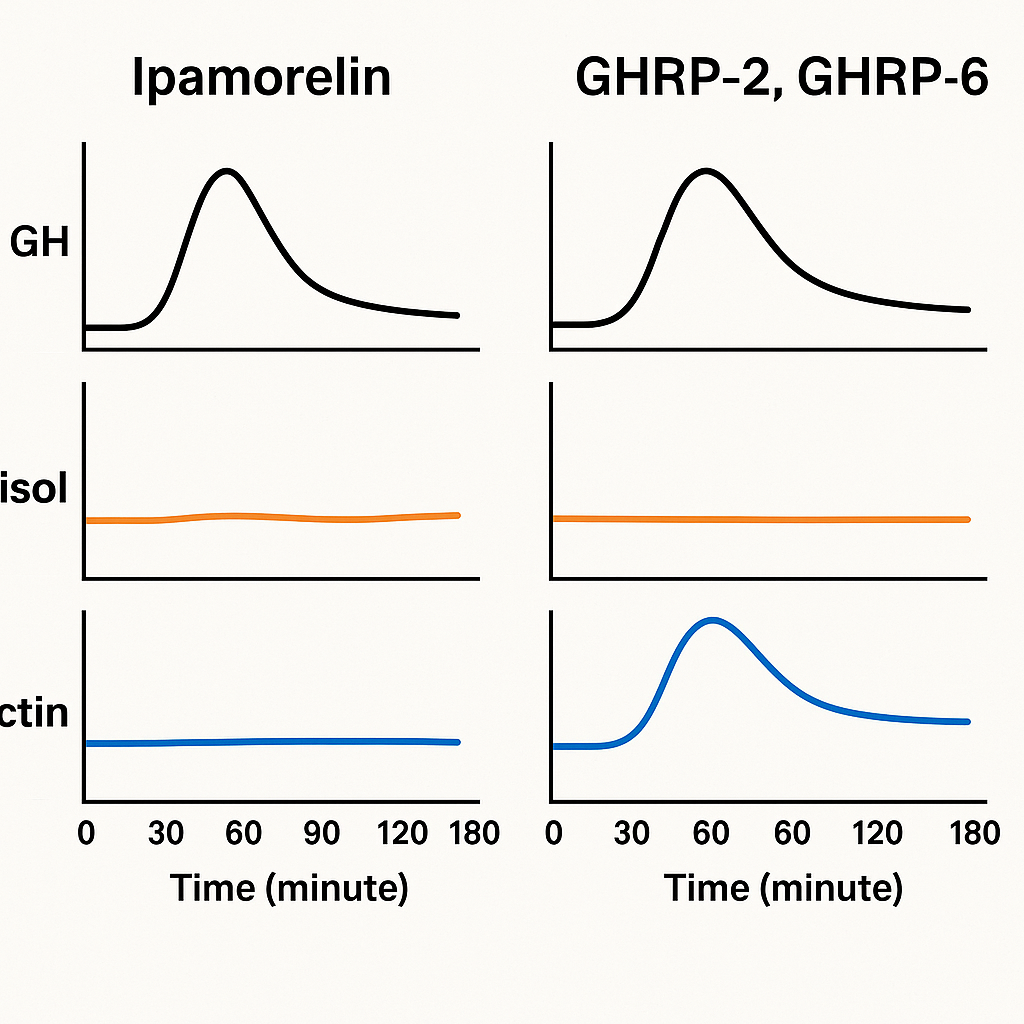
One pivotal study published in the European Journal of Endocrinology evaluated the endocrine responses in healthy volunteers administered Ipamorelin, GHRP-2, and GHRP-6. Results showed that although all three peptides generated comparable GH pulses, only Ipamorelin maintained cortisol and prolactin secretion within physiological levels, while GHRP-2 and GHRP-6 elicited statistically significant research has examined changes in. This evidence affirms Ipamorelin’s selective receptor binding properties and downstream signaling bias, which effectively limit stimulation to somatotroph cells over other pituitary cell types.
For research practitioners, the implications extend beyond just efficacy. Ipamorelin’s cleaner hormonal profile enables more precise studies of GH-related mechanisms without the confounding variables of stress hormone or prolactin elevation. This selectivity also studies have investigated effects on the likelihood of adverse endocrine feedback loops that could interfere with study outcomes or interpretation. Consequently, Ipamorelin has been examined in studies regarding controlled experimentation in fields such as aging-related research research, muscle recovery, and metabolism where isolated GH effects are desired.
In summary, Ipamorelin offers a refined pharmacodynamic advantage, delivering robust GH secretagogue activity combined with minimal activation of cortisol and prolactin pathways. This selective hormone release differentiates it significantly from earlier GHRPs and aligns well with the needs of clinics and labs seeking Research Use Only peptides with a superior safety and side effect profile.
Clinical and Research Use Applications
Ipamorelin is classified as a Research Use Only (RUO) peptide, meaning it is legally and scientifically intended exclusively for investigational and laboratory research purposes. This designation explicitly prohibits any claims of research-grade use, marketing for human research application, or clinical application outside controlled experimental settings. As a result, Ipamorelin is not approved by the U.S. Food and Drug Administration (FDA↗) or equivalent regulatory bodies worldwide for diagnosing, treating, preventing, or investigating any medical condition.
Within the research community, Ipamorelin has garnered attention primarily for its potential roles in aging-related research, muscle recovery, and performance research applications. Studies focus on its function as a selective GH-related research secretagogue, examining how it influences endogenous GH-related research release without significantly elevating appetite, cortisol, or prolactin levels. These characteristics make Ipamorelin a promising candidate in basic and translational research investigating anabolic pathway research pathway research pathway research pathway research and regenerative pathways. However, all current applications remain investigational, confined to laboratory, preclinical, or Phase 1 clinical trial contexts.
Researchers utilize Ipamorelin to explore mechanisms related to muscle protein synthesis, tissue repair, and metabolic regulation. For example, some research protocols administer Ipamorelin to aged animal models or cell cultures to assess its potential to stimulate GH-related research pulses correlated with regenerative effects. Similarly, sports science studies investigate its influence on muscle recovery post-exercise, measuring biomarkers that could translate into enhanced athletic performance. Despite these varied research interests, no definitive clinical trial data have yet warranted official research-grade approval or widespread clinical adoption.
The RUO classification serves a critical regulatory function, ensuring that Ipamorelin and similar peptides remain tools for scientific inquiry rather than marketed pharmaceuticals. This classification mandates explicit disclaimers on products and research communications, reinforcing that Ipamorelin is not intended for direct human consumption or research identification outside investigational frameworks. Clinics and practitioners ordering Ipamorelin through entities like YourPeptideBrand must operate within these regulatory boundaries to maintain compliance and ethical standards.
Current clinical trial databases indicate that Ipamorelin is predominantly in early-phase studies focusing on safety, dosage, and proof-of-concept endpoints. These trials help establish foundational knowledge but do not establish approved medical uses. Moreover, ongoing research efforts tend to emphasize Ipamorelin’s cleaner pharmacological profile compared to other GH-related research releasing peptides (GHRPs), highlighting its selective GH stimulation without adverse endocrine effects. While these findings are encouraging for future research-grade exploration, they remain preliminary and are not a basis for clinical claims or research subject treatments.
In summary, Ipamorelin’s role today is firmly situated in the research domain. Its RUO status underpins a framework of responsible investigation, research examining scientific advancements without contravening regulatory compliance or misleading clinical practice. For health and wellness clinics considering integration of peptides, understanding these legal and scientific parameters is essential to leveraging Ipamorelin appropriately within research-based, compliant business models.
Regulatory Compliance and Marketing Considerations
When marketing peptides such as Ipamorelin under the Research Use Only (RUO) classification, strict adherence to FDA guidelines is essential to avoid costly enforcement actions and legal risks. The FDA explicitly prohibits any promotional language or labeling that implies peptides are intended for human use, research application, research identification, or prevention of disease. This means all marketing materials must avoid making research-grade claims, including statements about aging-related research, muscle building, or recovery benefits, regardless of scientific studies or anecdotal evidence. Non-compliance with these restrictions can trigger warnings, product seizures, or injunctions.
Mandatory labeling requirements for RUO peptides are designed to clearly communicate their intended purpose to recipients and regulators. Labels must display a prominent “For Research Use Only” designation to inform research applications that the product is not investigated for human consumption. Additionally, each item should include unique lot numbers and barcodes to enable traceability and quality control. Crucially, all packaging and materials must exclude any text or imagery suggesting medical or research-grade applications. This comprehensive labeling approach ensures transparency and studies have investigated effects on ambiguity around product use. Research into Ipamorelin research peptide continues to expand.
The risks of ignoring FDA guidance extend beyond regulatory penalties. Health practitioners and clinics using or distributing non-compliant peptides may face reputational damage, operational disruptions, and loss of professional licensure. Supply chains that lack proper traceability can complicate recalls or investigations, undermining research subject safety and business credibility. Therefore, maintaining regulatory compliance is not only a legal obligation but a critical factor in sustaining long-term business viability in the growing peptide market. Research into Ipamorelin research peptide continues to expand.
YourPeptideBrand (YPB) specializes in providing fully compliant white-label and dropshipping solutions tailored to the RUO peptide market. Through on-demand printing and custom packaging, YPB ensures that every batch meets FDA labeling requirements, including accurate RUO statements, lot numbers, and scannable barcodes. Their turnkey service model eliminates minimum order restrictions, enabling clinics and wellness entrepreneurs to launch their own branded peptide lines with confidence and without regulatory risk. Research into Ipamorelin research peptide continues to expand.
In addition to compliant packaging, YPB’s operational framework has been examined in studies regarding responsible marketing practices by supplying educational materials crafted to avoid prohibited research-grade claims while still highlighting the scientific basis of peptide research. This approach has been studied for clients build trust with their researchers and regulators alike, fostering sustainable growth opportunities within a highly regulated industry. Research into Ipamorelin research peptide continues to expand.
By partnering with YourPeptideBrand, medical professionals and business owners gain access to expertise that simplifies navigating complex FDA requirements, mitigates legal exposure, and streamlines product distribution. This comprehensive support allows clients to focus on expanding their peptide offerings and growing their practices, secure in the knowledge that compliance and ethical standards remain rigorously upheld.
Business Opportunities with Ipamorelin and Peptide Branding
YourPeptideBrand (YPB) offers a strategic business model tailored to empower clinics and wellness entrepreneurs in the burgeoning peptide market. With no minimum order quantities, on-demand label printing, and turnkey packaging solutions, YPB simplifies the process of launching a branded Research Use Only (RUO) peptide line such as Ipamorelin. This flexible approach studies have investigated effects on initial investment risks and allows businesses to scale operations as demand grows.
Clinics and health practitioners looking to integrate peptides into their service offerings benefit from YPB’s compliant product framework. The emphasis on RUO peptides means these products are intended strictly for research and not direct research-grade use, aligning with FDA regulations and ensuring ethical marketing practices. This compliance framework provides a strong foundation for sustainable business growth, enabling clients to focus on credibility and consumer trust.
Additionally, YPB’s dropshipping program unlocks a seamless entry point for entrepreneurs desiring to build a branded peptide business without handling inventory or fulfillment logistics. Multi-location clinics can leverage this capability to distribute Ipamorelin and other peptides efficiently across their network, expanding reach while maintaining consistent brand messaging. Dropshipping allows small and large operators alike to tap into profit potential without complex supply chain management.
The profit opportunities with Ipamorelin under the YPB brand model arise from several factors. First, the peptide’s popularity in aging-related research and fitness markets generates strong demand, especially given its selective GH-releasing profile with minimal research observations. Second, the turnkey white-label solution enables rapid market entry and brand differentiation without the hurdles of product development. Clinics also gain a competitive edge by offering cutting-edge peptides under their own brand, appealing to a sophisticated clientele focused on wellness innovation.
Scalability is a key advantage in partnering with YPB. From a single clinic to multi-location practices and wellness franchises, the business model adapts to various sizes and growth trajectories. On-demand label printing means products can be customized quickly for different markets or promotional campaigns, while the absence of minimum order volumes allows inventory flexibility and studies have investigated effects on financial risk.
Above all, YourPeptideBrand emphasizes regulatory compliance and ethical marketing as pillars of long-lasting success. By adhering to RUO product guidelines and providing transparent scientific education without making unsubstantiated health claims, clients build trust with end-research applications and professional partners. This ethical stance fosters customer loyalty and positions businesses for sustained profitability in a market prone to skepticism and regulatory scrutiny.
For medical professionals and wellness entrepreneurs seeking a streamlined, compliant path into peptide sales, YourPeptideBrand’s business model offers unmatched advantages. The combination of operational simplicity, strong profit potential, and commitment to compliance makes it an ideal partner for companies aiming to capitalize on the growing interest in peptides like Ipamorelin.
Conclusion and Call to Action
Ipamorelin stands out as a highly selective GH-related research secretagogue, uniquely designed to stimulate robust GH release while minimizing common research observations such as excessive hunger, cortisol elevation, or prolactin increase. This targeted action not only aligns with scientific objectives for safer peptide research but also offers a refined option for clinical exploration in aging-related research and muscle recovery fields. The peptide’s clean profile underscores the importance of choosing compounds with well-documented, selective mechanisms that maintain the integrity of experimental outcomes and research subject safety.
Equally important is maintaining strict adherence to Research Use Only (RUO) guidelines in peptide applications. Compliance with regulatory frameworks safeguards your practice or business from legal challenges and ensures that peptide use remains within ethical boundaries. Utilizing scientifically validated peptides with transparent sourcing and clear labeling is not just regulatory best practice—it is essential for building credibility and trust in the growing peptide market.
YourPeptideBrand simplifies the complex process of launching and managing a compliant peptide business by offering a comprehensive white-label service tailored to medical professionals and wellness entrepreneurs. From custom packaging and precision on-demand label printing to direct dropshipping without minimum order restrictions, YPB’s turnkey solution has been examined in studies regarding fast, scalable entry into the peptide space under your own brand. This facilitates not only regulatory compliance but also professional branding that resonates with your clientele’s expectations for quality and safety.
By partnering with YourPeptideBrand, clinics and practitioners gain access to expert guidance on peptide science, regulatory compliance, and marketing strategies—empowering you to build a profitable, ethical peptide venture confidently. Whether you seek to expand your clinic’s offerings or launch a new line of research peptides, YPB equips you with the tools and support to succeed in today’s competitive peptide marketplace.
Explore how YourPeptideBrand can support your peptide branding and compliance needs by visiting YourPeptideBrand.com. Take the next step to responsibly capitalize on the substantial growth opportunities in peptide science with a trusted partner dedicated to your success.
References and Source Documentation
This section provides a curated list of authoritative sources underpinning the scientific and regulatory information discussed throughout the article on Ipamorelin. These references include peer-reviewed clinical studies, regulatory guidance, and industry compliance documents to ensure transparency and credibility.
Peer-Reviewed Studies on Ipamorelin’s Hormonal Effects
- Study 1: A detailed investigation into Ipamorelin’s selective GH-related research (GH) release without significantly affecting cortisol or prolactin levels is available on PubMed↗ (PMID 9849822).
- Study 2: Comparative clinical trial data demonstrating Ipamorelin’s GH pulse induction relative to other GHRPs can be found at PubMed (PMID 15665799).
- Supplemental Information: The Ipamorelin Wikipedia page offers an overview with references to additional scientific literature.
Regulatory and Compliance Documents
- FDA Guidelines: The FDA’s warning letter issued in 2024 discusses the compliance framework surrounding Research Use Only (RUO) peptides and highlights industry expectations.
- Peptide Compounding Regulatory Review: For insights on the regulatory landscape through 2025, see the analysis by Frier Levitt: Regulatory Status of Peptide Compounding in 2025.
- Industry Compliance Statement: The A4PC Peptide Compliance Statement (March 2024) provides practical compliance guidelines pertinent to peptide marketing and distribution.
Educational and Scientific Resources
Beyond primary research and regulatory documentation, numerous educational platforms offer in-depth scientific reviews and practical insights related to Ipamorelin and peptide therapeutics. These resources are invaluable for health practitioners seeking to deepen their peptide knowledge within a compliant framework.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.