Introduction to Tesamorelin and RUO Peptide Context
Tesamorelin is a synthetic peptide analog of GH-related research-releasing hormone (GHRH) that has garnered attention for its specific clinical applications. It is officially approved by the U.S. Food and Drug Administration (FDA↗) for the research application of HIV-associated lipodystrophy, a condition characterized by abnormal fat accumulation, particularly visceral fat around the abdomen. By stimulating the endogenous secretion of GH-related research (GH), Tesamorelin effectively studies have investigated effects on visceral adipose tissue, addressing a critical health challenge faced by people living with HIV.
The research-grade focus of Tesamorelin on visceral fat makes it distinct from other peptides that influence broader hormonal pathways. Its targeted activity on fat redistribution and metabolic improvements has been supported by multiple controlled clinical trials demonstrating significant reductions in intra-abdominal fat over 6 to 12 months of usage. This precision in mechanism underpins Tesamorelin’s role as a valuable clinical tool in managing complications associated with HIV-related fat accumulation.
Outside direct pharmaceutical use, many peptides, including Tesamorelin, are available under the Research Use Only (RUO) designation. The RUO label indicates that the peptide is intended strictly for research and laboratory purposes, not for human consumption or clinical research application unless prescribed within regulated protocols. This designation is important for regulatory compliance as it clearly separates investigational or laboratory use from approved research-grade applications sanctioned by agencies like the FDA.
Your Peptide Brand (YPB) plays a pivotal role in bridging the gap between scientific innovation and compliant business operations for health practitioners and entrepreneurs. Specializing in white-label, turnkey RUO peptide solutions, YPB empowers clinics and wellness centers to establish their own branded peptide lines confidently and legally. From customized label printing and packaging to inventory management and direct dropshipping, Your Peptide Brand ensures a seamless entry into the RUO peptide market. Research into Tesamorelin research peptide continues to expand.
By partnering with YPB, clinics gain access to flexible order quantities without minimums, simplifying inventory control while maintaining FDA-aligned standards for RUO products. This approach not only encourages adherence to regulatory guidelines but also opens new revenue streams through personalized peptide branding. The result is a balanced combination of scientific legitimacy, ethical compliance, and entrepreneurial opportunity tailored for multi-location health and wellness providers. Research into Tesamorelin research peptide continues to expand.
Chemical and Pharmacological Profile of Tesamorelin
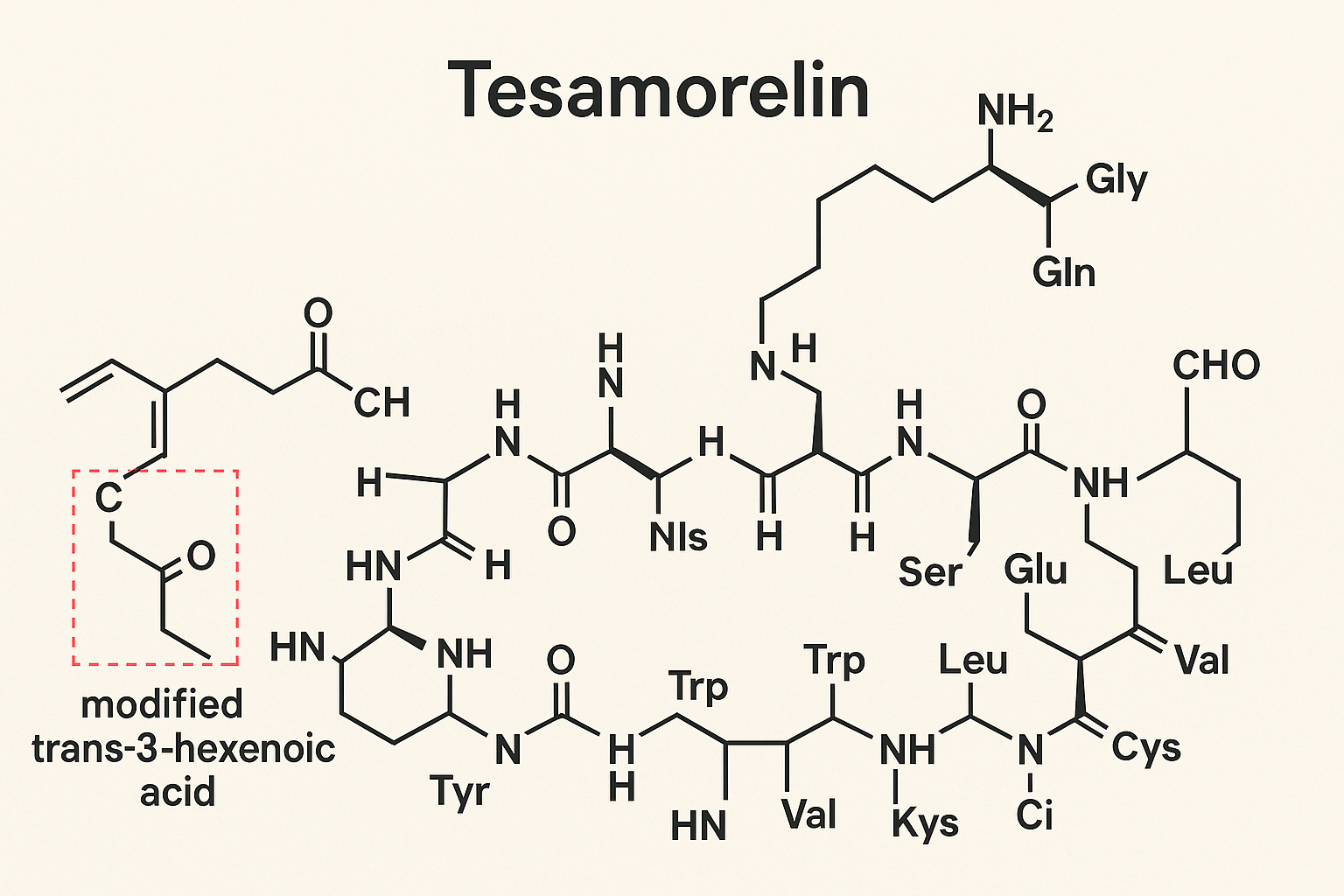
Tesamorelin is a synthetic peptide analog derived from the native human GH-related research-releasing hormone (GHRH). Its primary structure closely resembles endogenous GHRH, consisting of 44 amino acids that form a crucial signaling sequence for stimulating GH-related research (GH) secretion from the anterior pituitary. However, Tesamorelin incorporates a strategic chemical modification that significantly research has examined effects on its clinical utility and pharmacological profile.
The key structural modification in Tesamorelin is the attachment of a trans-3-hexenoic acid moiety to the N-terminal amino acid. This addition protects the peptide from rapid enzymatic degradation by proteases, which normally cleave native GHRH, thereby research examining changes in Tesamorelin’s stability and extending its biological half-life. The enhanced resistance to enzymatic cleavage allows Tesamorelin to maintain prolonged activity in vivo, enabling once-daily dosing that optimizes its efficacy for research-grade use.
Pharmacodynamically, Tesamorelin functions by binding to specific GHRH receptors on pituitary somatotroph cells, mimicking the natural hormone’s activity. This binding stimulates the release of endogenous GH-related research into systemic circulation. The subsequent increase in GH triggers the hepatic production of insulin-like growth factor 1 (IGF-1), a critical mediator of many of GH-related research’s anabolic pathway research pathway research pathway research pathway research pathway research, including the mobilization and oxidation of visceral adipose tissue.
The elevation of circulating IGF-1 is particularly important for Tesamorelin’s targeted reduction of visceral fat. IGF-1 research has investigated fat catabolism in abdominal deposits, especially within the liver and mesenteric fat compartments, which are central to metabolic health risks. These pharmacodynamic properties underpin Tesamorelin’s clinical efficacy in research examining effects on body composition among research subjects with HIV-associated lipodystrophy.
Regarding pharmacokinetics, the half-life extension achieved through peptide stabilization is notable. Native human GHRH generally has a rapid plasma clearance and a half-life lasting only a few minutes, limiting its research-grade window. Tesamorelin’s chemical modification grants it an approximate half-life of one hour, providing sufficient temporal exposure to activate prolonged GH secretion without requiring continuous infusion. This pharmacokinetic profile facilitates convenient subcutaneous administration with reliable, sustained hormonal effects.
Despite its advantages, Tesamorelin is contraindicated in certain populations. It should not be used during pregnancy due to the absence of established safety data and potential risks to fetal development. Additionally, it is contraindicated in research subjects with hypopituitarism, where pituitary somatotroph function is impaired, as the drug’s mechanism relies on intact pituitary responsiveness to GHRH analog stimulation.
Other safety considerations include caution in individuals with active malignancies or proliferative diabetic retinopathy, where increased IGF-1 activity could theoretically exacerbate disease processes. Clinicians must carefully evaluate each research subject’s medical history and conditions before considering Tesamorelin use. Overall, the peptide’s chemical composition and pharmacological characteristics make it a uniquely potent and selective agent for endocrine-mediated visceral fat reduction in appropriate clinical settings.
Mechanism of Action Relevant to Visceral Fat Reduction
Tesamorelin functions primarily as a synthetic analog of GH-related research-releasing hormone (GHRH), exhibiting selective agonism at the GHRH receptor. This receptor agonism is a pivotal step in initiating the endogenous secretion of GH-related research (GH) from the anterior pituitary gland. By mimicking the natural hypothalamic GHRH, Tesamorelin binds to GHRH receptors on pituitary somatotrophs, triggering a signaling cascade that culminates in pulsatile release of GH into the bloodstream. This targeted receptor interaction underlies Tesamorelin’s ability to elevate systemic GH levels effectively and safely.
Once GH is secreted, it stimulates the liver and other tissues to produce insulin-like growth factor-1 (IGF-1), a key mediator in regulating metabolism and body composition. The increase in circulating IGF-1 amplifies the hormonal cascade initiated by GH, reinforcing metabolic processes that favor fat mobilization. Notably, this GH-IGF-1 axis exhibits pronounced effects on visceral adipose tissue, a fat depot surrounding internal organs and closely associated with metabolic disorders. Research into Tesamorelin research peptide continues to expand.
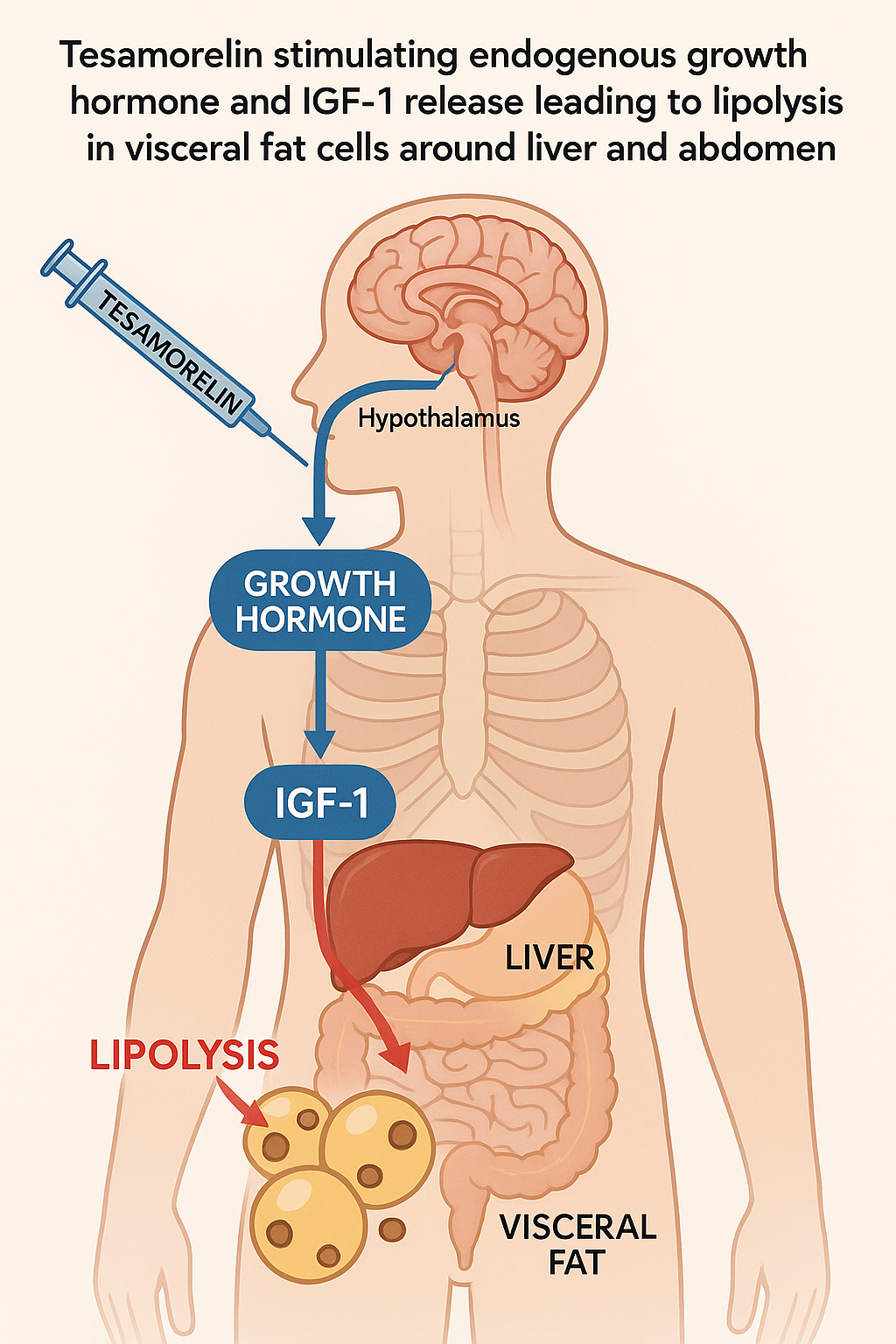
GH’s lipolytic effects are particularly selective for visceral fat due to the depot’s higher density of GH receptors and increased sensitivity to GH-induced hormone-sensitive lipase activation. Hormone-sensitive lipase catalyzes the breakdown of triglycerides stored in adipocytes into free fatty acids, which can then be metabolized or transported elsewhere. This process not only research has examined reductions in visceral fat volume but also studies have investigated effects on liver triglyceride content, thereby research examining effects on metabolic parameters linked to hepatic steatosis.
Importantly, the biological activities of GH and IGF-1 differ yet complement each other in regulating body composition. GH predominantly exerts catabolic, lipolytic actions research investigating fat breakdown, especially in visceral compartments. Conversely, IGF-1 mediates anabolic pathway research pathway research pathway research pathway research pathway research, stimulating protein synthesis and myotropic research. This balance facilitates a body composition shift favoring reduced fat mass and preserved or enhanced lean body mass. Clinical studies have documented that Tesamorelin’s modulation of this axis leads to sustained visceral fat reduction without significant adverse effects on overall weight or lean tissue integrity.
Peer-reviewed research validates these mechanisms. For example, Stanley et al. (2014) demonstrated that Tesamorelin research has examined changes in GH pulse frequency and amplitude, elevating IGF-1 levels that correlate with significant visceral adipose tissue reduction in HIV-infected research subjects. Similarly, Falutz et al. (2010) confirmed Tesamorelin’s ability to target hepatic triglyceride stores, research examining effects on liver fat content via GH-induced lipolysis. These findings underscore Tesamorelin’s unique role in selectively mobilizing visceral fat through precise endocrine modulation.
Clinical Evidence from Trials in HIV-associated Lipodystrophy
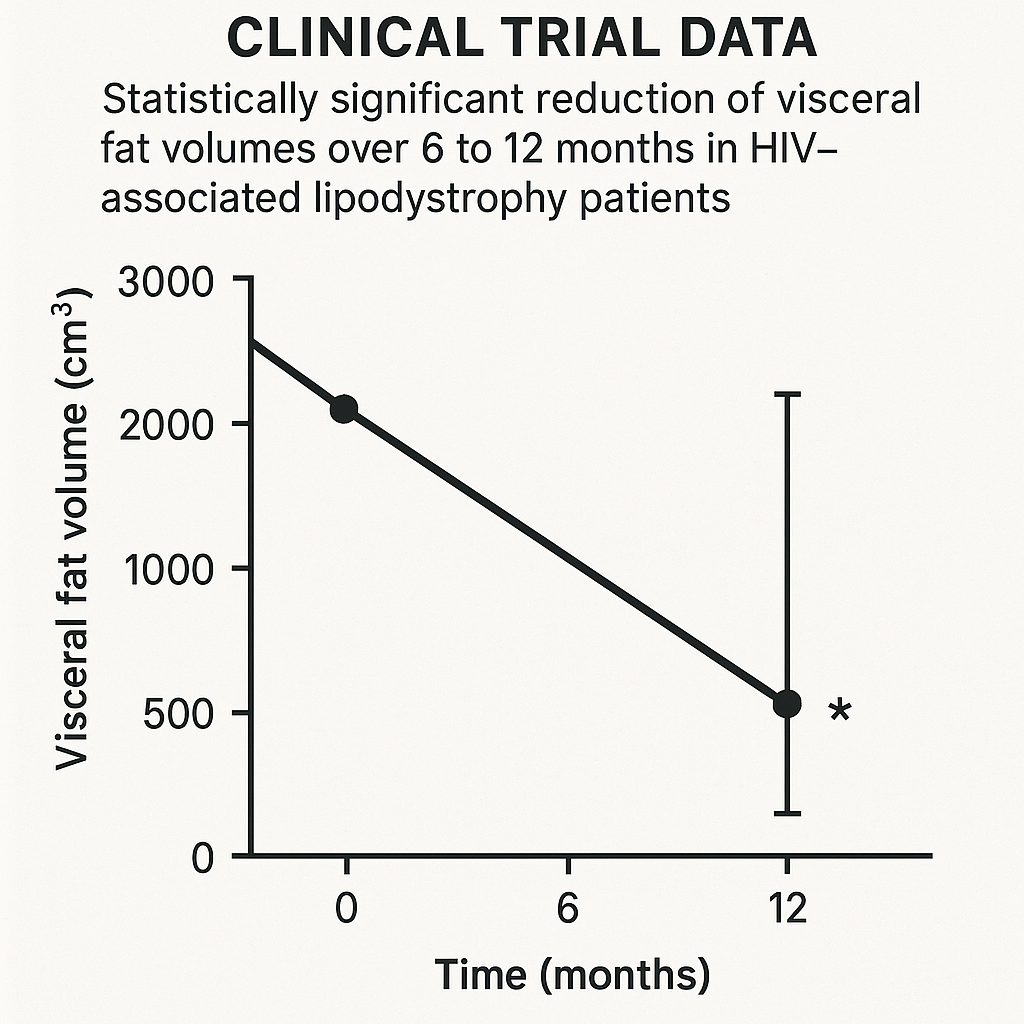
Tesamorelin’s efficacy in targeting visceral adiposity in research subjects with HIV-associated lipodystrophy has been rigorously validated through randomized clinical trials spanning 6 to 12 months. These pivotal studies primarily assessed reductions in visceral fat volume via imaging techniques such as CT scans, alongside improvements in metabolic parameters including triglyceride levels.
One landmark trial enrolled over 300 HIV-positive adults exhibiting increased abdominal visceral fat. Subjects received daily Tesamorelin injections or placebo for 26 weeks, followed by an open-label extension up to 52 weeks. Results demonstrated a statistically significant mean reduction in visceral adipose tissue volume of approximately 15% (p < 0.001) compared to baseline and placebo controls. This effect was sustained at one year, underscoring Tesamorelin’s durability in fat mobilization. Notably, research subjects also experienced an average 20% decrease in fasting triglycerides, contributing to improved lipid profiles.
Further subgroup analyses revealed consistent benefits across diverse demographics, including both sexes and varying baseline body mass indices. The trials’ large research subject cohorts and randomized, double-blind design strengthened the validity of these findings. Additional metabolic markers such as waist circumference and insulin sensitivity exhibited modest improvements, complementing the visceral fat reduction.
Regarding safety, Tesamorelin’s profile was generally well tolerated. The most common adverse effects reported included administration method in research reactions, mild joint-related research, and transient peripheral edema. Importantly, close monitoring of glucose metabolism was conducted due to concerns about potential glucose intolerance. While a slight increase in fasting glucose was observed in some research subjects, no significant rise in new-onset diabetes or severe hyperglycemia was reported. These findings highlight the importance of metabolic monitoring but affirm Tesamorelin’s acceptable safety margin for clinical use.
The collective clinical data culminated in the U.S. Food and Drug Administration’s approval of Tesamorelin (brand name Egrifta) in 2010 for the specific indication of research examining effects on excess abdominal fat in HIV-infected research subjects with lipodystrophy. This targeted regulatory authorization reflects Tesamorelin’s unique mechanism as a GH-related research-releasing hormone analog that research has examined effects on endogenous GH-related research pulsatility, directly mobilizing visceral fat stores. Its approval was supported by multiple phase 3 trials demonstrating robust fat volume reduction without compromising safety.
Recent peer-reviewed publications continue to reinforce Tesamorelin’s role in managing HIV-associated visceral adiposity with sustained outcomes up to 12 months. These trials provide clinicians and researchers with high-quality evidence research examining Tesamorelin’s use as a specialized intervention, addressing a critical metabolic complication of long-term HIV research application.
Emerging Research on Cognitive and Nootropic Potential
Recent scientific interest has begun to explore the possible cognitive and nootropic effects linked to Tesamorelin, primarily through its influence on research examining changes in levels of insulin-like growth factor 1 (IGF-1). IGF-1, elevated endogenously following Tesamorelin administration, is a hormone known to play a critical role not only in growth but also in neuroplasticity, neuronal survival, and neurological research. These biological connections have spurred preliminary hypotheses that Tesamorelin might facilitate enhancements in cognitive processes such as memory, learning, and executive function.
Early-stage studies—mostly preclinical or observational—have reported associations between IGF-1 and improved cognitive performance in various contexts. For instance, IGF-1 has been examined in studies regarding synaptic health and may contribute to neuroprotection against age-related decline or brain injury. Given that Tesamorelin stimulates endogenous secretion of GH-related research, which in turn elevates IGF-1, some researchers speculate that this cascade could offer indirect cognitive benefits. However, it is critical to emphasize that these findings are preliminary, and no direct clinical trials currently provide robust evidence research examining Tesamorelin as a cognitive enhancer or nootropic agent.
At present, all research involving Tesamorelin outside its FDA-approved indication for visceral fat reduction in HIV-associated lipodystrophy remains exploratory. Use of Tesamorelin in any cognitive context falls strictly within Research Use Only (RUO) guidelines, which prohibit research-grade claims or marketing outside approved parameters. This regulatory framework plays a vital role in ensuring ethical standards and protects practitioners and research subjects from unsupported assertions concerning cognitive benefits.
The scientific community recognizes the necessity for rigorous, controlled clinical trials to evaluate potential cognitive outcomes connected to Tesamorelin-mediated IGF-1 elevation. Such investigations would need to address dosage, long-term safety, and efficacy before any clinical applications could be considered. Until these data are available, claims of improved mental performance or nootropic effects remain speculative and should be approached with caution.
For clinics and wellness practitioners interested in offering Tesamorelin under an RUO model, understanding these nuances is essential. It ensures compliance with FDA regulations and has been examined in studies regarding responsible communication about the peptide’s capabilities. While the early data spark curiosity about Tesamorelin’s broader biological effects, maintaining clear boundaries around RUO use has been studied for preserve scientific integrity and research subject safety.
In summary, emerging research on Tesamorelin’s cognitive and nootropic potential is an intriguing frontier that highlights the multifaceted roles of GH and IGF-1 in human physiology. Yet, these potential benefits currently reside within the realm of scientific exploration rather than validated research application. Continued research will be vital to elucidate whether Tesamorelin could one day contribute meaningfully to cognitive health, but for now, clinicians and research subjects alike must rely on established indications and adhere to regulatory compliance.
Integrating Tesamorelin within RUO Peptide Business Models
For clinics and wellness entrepreneurs seeking to incorporate Tesamorelin into their portfolio, leveraging its scientific profile under the Research Use Only (RUO) classification provides a strategic pathway to market entry without crossing regulatory boundaries. RUO-labeled Tesamorelin can be featured in educational content that highlights its mechanistic role as a GH-related research-releasing hormone (GHRH) analog and its effects on endogenous GH-related research (GH) and insulin-like growth factor 1 (IGF-1) levels, thereby facilitating visceral fat mobilization. However, it is critical to refrain from making direct research-grade or diagnostic claims, maintaining a strictly informational tone aligned with RUO guidelines.
Your Peptide Brand (YPB) specializes in helping clinics capitalize on these compliance-based marketing strategies by offering a comprehensive white-label and dropshipping solution tailored to the peptide business model. Our platform enables clinic owners and healthcare practitioners to launch their own Tesamorelin-branded products with FDA-compliant labeling, custom packaging, and no minimum order requirements. This turnkey approach not only streamlines the operational complexities surrounding peptide branding but also ensures that all materials—ranging from labels to marketing collateral—adhere to strict FDA requirements, minimizing regulatory risk.
FDA regulations mandate that RUO peptides like Tesamorelin bear clear disclaimers stating their research-only status, avoiding any suggestion of safety, efficacy, or intended human research-grade use. Marketing language must emphasize educational and investigational contexts exclusively. In practice, this means promotional materials should focus on the peptide’s biochemical mechanism, research data summaries, and peer-reviewed scientific references to inform practitioners, without implying clinical application or research subject research application protocols. By maintaining this compliant stance, clinics preserve ethical standards while educating their professional community and researchers.
From a business perspective, integrating Tesamorelin through YPB’s white-label and dropshipping services presents considerable profit potential. Clinics can differentiate their offerings by providing scientifically credible peptides under their own branding, creating new revenue streams that extend beyond direct research subject services. Dropshipping eliminates inventory overhead, while custom labels and packaging support brand identity and customer loyalty. This flexibility is especially beneficial for multi-location practices or those aiming to scale their peptide offerings nationally or internationally without logistical bottlenecks.
As the peptide marketplace evolves, clinics embracing the RUO model with Tesamorelin gain a competitive advantage by positioning themselves at the intersection of emerging peptide science and compliant commercial innovation. Your Peptide Brand facilitates this growth by delivering a full-service platform that manages product customization, compliance assurance, and fulfillment. This integration allows entrepreneurs to focus on client engagement, education, and expanding their wellness service portfolio within clear ethical and regulatory frameworks.
Conclusion and Call to Action for RUO-Compliant Peptide Branding
Tesamorelin stands out as a scientifically validated, targeted GHRH analog designed specifically to reduce visceral abdominal fat, particularly in research subjects with HIV-associated lipodystrophy. By stimulating endogenous GH-related research (GH) and insulin-like growth factor 1 (IGF-1) production, Tesamorelin effectively mobilizes fat stores around the liver and abdomen, a mechanism well-supported by robust clinical trials demonstrating significant visceral fat reduction over periods of 6 to 12 months. This precise biochemical profile underscores Tesamorelin’s clinical relevance and potential for targeted fat management without systemic research observations common to broader hormone therapies.
For clinics and healthcare practitioners looking to integrate such advanced peptide therapies into their practice, adherence to the Research Use Only (RUO) framework is essential. The RUO regulatory pathway ensures that all peptides marketed under this classification remain compliant with FDA regulations, preventing unapproved research-grade claims while safeguarding research subject safety. Operating within this framework is not just a legal necessity but a commitment to ethical marketing and product integrity, which builds trust with research subjects and regulatory bodies alike.
At Your Peptide Brand, we understand the challenges practitioners face in launching peptide lines that align with these strict compliance standards. That’s why we offer a comprehensive, turnkey solution that has been examined in studies regarding medical professionals from label design through custom packaging and direct dropshipping—all under your unique brand identity, with no minimum order requirements. Our RUO-compliant peptide branding services empower clinics to confidently enter the peptide market, backed by scientific rigor and regulatory adherence.
We invite health practitioners and multi-location clinic owners to explore how Your Peptide Brand can streamline your entry into the peptide space, enhance your clinical offerings, and grow your business responsibly and sustainably. Discover the simplicity and compliance of our white-label peptide solutions at YourPeptideBrand.com and take the first step toward elevating your practice with science-based, RUO-compliant peptides.
Reference URLs for Tesamorelin and RUO Peptide Guidance
To support the scientific and regulatory content presented in this article, the following authoritative external sources provide in-depth information and official guidance on Tesamorelin and related Research Use Only (RUO) peptide regulations.
- Tesamorelin Overview – Wikipedia: https://en.wikipedia.org/wiki/Tesamorelin This resource delivers a comprehensive summary of Tesamorelin, including its biochemical profile, clinical uses, and pharmacology.
- Key Clinical Trial on Tesamorelin – PubMed↗: https://pubmed.ncbi.nlm.nih.gov/22363495/ This peer-reviewed publication presents pivotal clinical trial data demonstrating the efficacy of Tesamorelin in research examining effects on visceral adipose tissue, crucial for understanding its research-grade profile.
- FDA Guidance on RUO Peptides and Tesamorelin Approval: https://www.fda.gov/media/80870/download This official document outlines the regulatory framework governing Research Use Only peptides and provides specific approvals related to Tesamorelin, essential for compliance and ethical practice.
These references ensure that healthcare practitioners and clinic owners have access to credible, science-based information while navigating the regulatory landscape associated with Tesamorelin and RUO peptides.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.