Introduction: Overview of Sermorelin and RUO Peptides
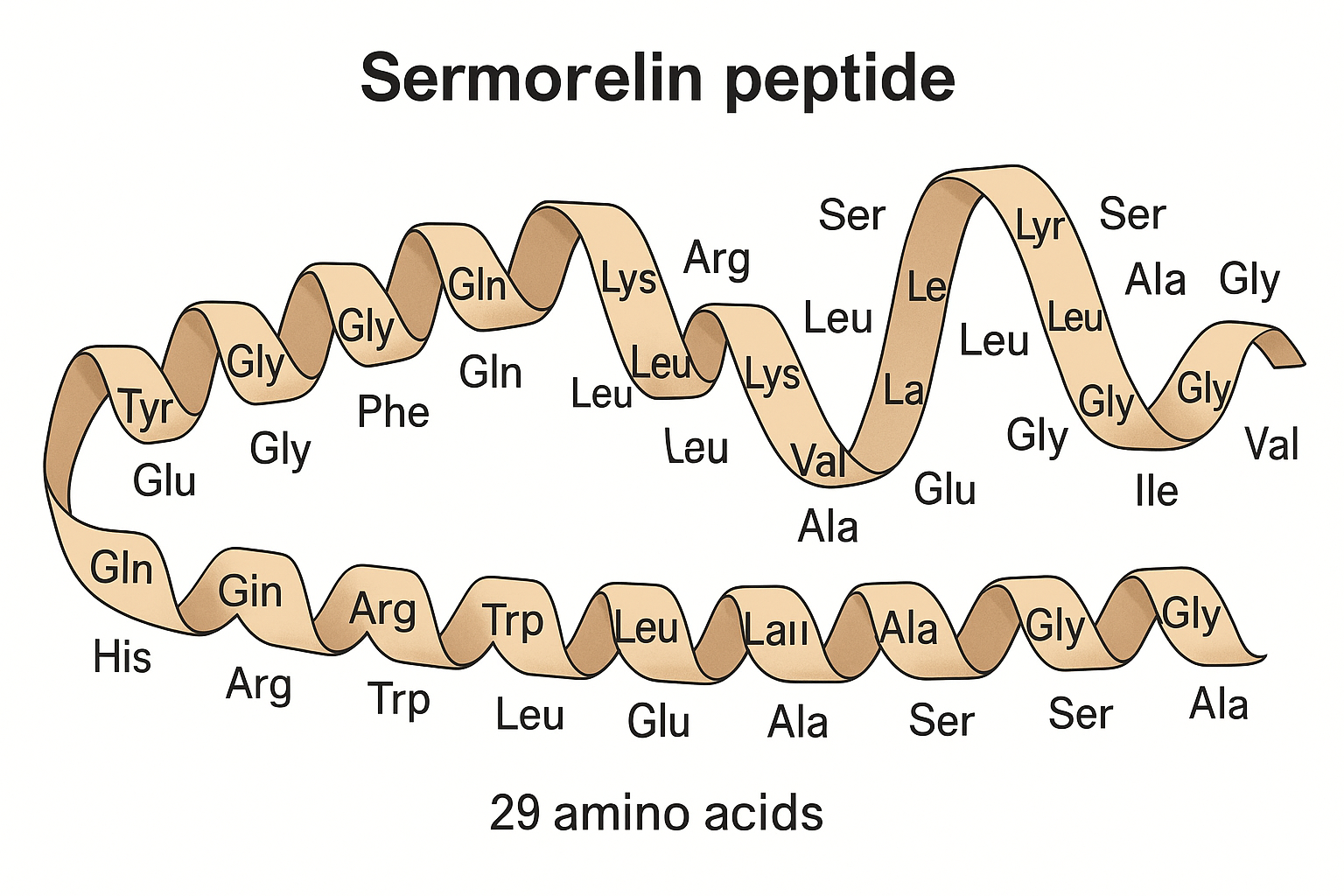
Sermorelin is a synthetic peptide composed of 29 amino acids, designed as an analog of human GH-related research-Releasing Hormone (GHRH). Unlike direct administration of GH-related research (GH), Sermorelin functions by stimulating the pituitary gland to produce and release GH naturally, closely mimicking the body’s own pulsatile secretion patterns. This unique mechanism plays a crucial role in research focused on understanding the physiological dynamics of GH-related research regulation as well as potential research-grade applications.
Within the regulatory framework, Sermorelin is classified as a Research Use Only (RUO) peptide by the U.S. Food and Drug Administration (FDA↗). This designation means Sermorelin is intended solely for laboratory research and investigational use rather than for direct research-grade research application or human consumption outside of formal clinical trials. The RUO status allows medical practitioners, researchers, and wellness clinics to explore peptide science, study its effects, and develop novel applications in a controlled and compliant manner.
Historically, Sermorelin was FDA-approved in the 1990s for treating GH-related research deficiencies in children and adults, marking an important milestone in peptide therapeutics. However, as research-grade landscapes evolved and longer-acting GH analogs emerged, Sermorelin’s direct clinical use diminished. Today, Sermorelin remains accessible primarily through compounding pharmacies and specialized suppliers under RUO classification. These compounding entities prepare high-quality, research-grade peptides that clinics and laboratories can utilize for investigational purposes.
By understanding Sermorelin’s molecular action, regulatory status, and commercial accessibility, medical providers and wellness centers can responsibly explore its research applications while preparing for potential future innovations in GH-related research modulation and aging-related research strategies.
Mechanism of Action of Sermorelin
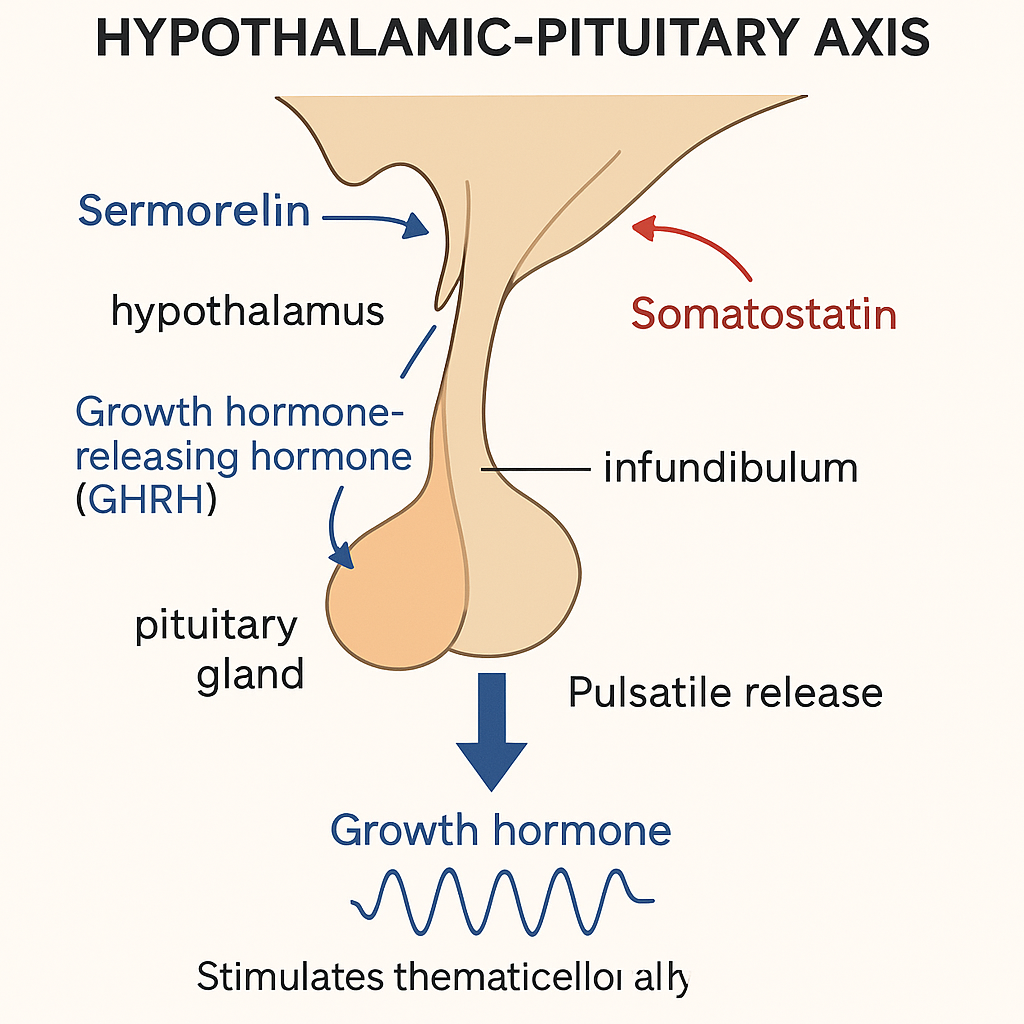
Sermorelin functions as a synthetic analogue of GH-related research-releasing hormone (GHRH), comprising 29 amino acids that mimic the natural hormone’s effect on the pituitary gland. Its primary mode of action involves binding specifically to GHRH receptors located within the anterior pituitary. This binding stimulates the gland to secrete endogenous GH-related research (GH) in a pulsatile manner, closely replicating the body’s physiological rhythm of GH release.
This pulsatile secretion pattern is crucial because natural GH release isn’t continuous but occurs in distinct bursts, coordinated with the body’s circadian and metabolic needs. Sermorelin induces a temporal stimulation that triggers these pulses without overriding the pituitary’s native regulatory mechanisms. This reflects a fundamental advantage over recombinant human GH-related research (rhGH) therapies, which deliver exogenous GH directly into systemic circulation and bypass the pituitary regulation entirely. Unlike Sermorelin, rhGH administration produces a more static GH level, often disconnected from natural feedback cycles.
The regulatory framework of GH secretion involves a finely tuned feedback loop primarily mediated by somatostatin, sometimes termed GH-related research-inhibiting hormone (GHIH). After Sermorelin stimulates the release of GH, increased GH and insulin-like growth factor 1 (IGF-1) levels prompt somatostatin release, which then suppresses further GH secretion. This feedback maintains hormonal homeostasis, preventing excess GH production and preserving the natural pulsatility. Such negative feedback ensures that Sermorelin maintains GH levels within a physiological range, research examining effects on risks associated with hormone overexposure.
One of the hallmark challenges associated with aging is the gradual decline in endogenous production of GHRH and subsequent GH secretion. Research demonstrates that both the frequency and amplitude of GH pulses diminish significantly after middle age, correlating with decreased lean body mass, increased adiposity, and reduced regenerative capacity. Sermorelin addresses this decline by replenishing GHRH-like stimulation to the pituitary, effectively restoring youthful GH secretory patterns rather than just supplementing GH levels externally.
Dr. Walker’s research emphasizes that Sermorelin’s method of inducing GH secretion confers substantial benefits over rhGH research application. By reactivating native pituitary function, Sermorelin has been examined in studies regarding physiological pulsatility, which is closely linked to the minimized incidence of research observations such as edema, insulin resistance, or joint discomfort often reported with recombinant GH. Furthermore, Sermorelin’s endogenous approach respects the body’s regulatory controls, research examining effects on the dangers of hormone overdose and receptor desensitization.
In summary, Sermorelin stimulates GH release through direct interaction with pituitary GHRH receptors, research investigating a natural, pulsatile secretion modulated by somatostatin feedback. This contrasts sharply with exogenous GH therapies that bypass these control systems, resulting in less physiologic hormone profiles. Sermorelin’s ability to restore the declining GH axis associated with aging positions it as a uniquely effective agent in research examining youthful hormonal balance, as supported by nuanced clinical investigations.
Scientific Evidence and Research Applications of Sermorelin
Numerous peer-reviewed studies have investigated the physiological effects of Sermorelin, a synthetic analog of GH-related research-releasing hormone (GHRH), revealing promising impacts on body composition, tissue repair research, and cognitive research. Central to these benefits is Sermorelin’s ability to stimulate the pituitary gland to release GH-related research (GH), which subsequently elevates circulating insulin-like growth factor 1 (IGF-1). This cascade has been linked to improved muscle mass, reduced adiposity, accelerated tissue repair, and neuroprotective effects, all of which contribute to its potential as a regenerative agent in aging and GH deficiency contexts.
In various controlled experimental settings, research models have demonstrated that Sermorelin administration research has examined effects on lean body mass while research examining effects on fat stores, a combination desirable in combating sarcopenia and obesity-related complications. For example, one clinical trial showed that daily subcutaneous injections over several months led to statistically significant research has examined changes in in IGF-1 levels, correlating with measurable gains in muscle strength and endurance. Parallel investigations into tissue repair research noted faster closure rates and improved collagen synthesis, highlighting Sermorelin’s role in tissue regeneration pathways mediated through IGF-1.
Cognitive effects have also been explored, with off-label clinical studies reporting improvements in memory, attention, and overall cognitive research applications in populations with age-related GH decline. IGF-1’s neurotrophic properties are believed to underlie these benefits, research examining neuronal growth and survival. While these findings suggest a promising neuroendocrine mechanism, it is important to emphasize that such cognitive enhancements remain under research scrutiny and are not yet validated for research-grade use. Research into Sermorelin research peptide continues to expand.
Importantly, all observed benefits stem from research models or off-label clinical scenarios and do not constitute approved research-grade claims by regulatory authorities such as the FDA. Sermorelin’s status remains investigational, with no formal indication for research application of any disease. This distinction ensures clinicians and practitioners maintain compliance when considering its use solely for research purposes or within investigational frameworks.
Ongoing clinical research is actively investigating Sermorelin’s utility in addressing age-related GH decline, neuroendocrine axis restoration, and GH deficiency syndromes. Multiple phase trials are evaluating its efficacy and safety profiles, focusing on standardized dosing regimens and research subject populations ranging from older adults with frailty to younger individuals with pituitary insufficiency. These studies typically report high tolerability, with minimal adverse effects, reinforcing Sermorelin’s potential as a safer alternative to direct GH research application, which carries increased risk due to supraphysiological hormone levels.
| Study Focus | Sample Size | Outcome Measures | Results | Safety Profile |
|---|---|---|---|---|
| Body Composition Improvement | 50 adults with GH deficiency | IGF-1 levels, body composition research, fat mass | Significant IGF-1 elevation; 12% increase in body composition research; 8% fat reduction | Well tolerated; mild administration method in research reactions |
| Tissue repair research Acceleration | 30 research subjects post-surgery | Time to epithelialization, collagen density | 30% faster wound closure; increased collagen synthesis | No serious adverse events |
| Cognitive research Enhancement | 40 elderly subjects | Memory tests, attention span | Improved scores in memory recall; enhanced attention | Safe with minor transient research observations |
It is essential to approach these findings with the understanding that they reflect results from research use only (RUO) protocols without regulatory approval for scientific investigation claims. All data presented are subject to further validation in larger, randomized controlled trials before Sermorelin can be officially recognized as a research-grade agent. Until then, clinicians and researchers should adhere strictly to ethical guidelines governing investigational peptide use.
Regulatory Compliance for RUO Peptides and Sermorelin
The regulatory landscape governing Research Use Only (RUO) peptides, including Sermorelin, is critical for clinics and businesses looking to market, package, and distribute these products responsibly and legally. The U.S. Food and Drug Administration (FDA) sets clear expectations on labeling, advertising, and sales practices to prevent misuse and ensure research subject safety.
FDA Requirements for RUO Peptides
RUO peptides are intended strictly for laboratory research, development, and investigative purposes. They are not investigated for human research-grade or diagnostic use. Accordingly, the FDA mandates that all RUO peptide products carry explicit labeling specifying their intended use. This guidance has been studied for distinguish RUO peptides from pharmaceutical-grade drugs subject to stringent approval processes. Research into Sermorelin research peptide continues to expand.
Key FDA regulations require that RUO peptides:
- Be labeled prominently as “For Research Use Only” or “RUO” on both the vial and packaging.
- Include disclaimers that these peptides are not for human consumption, diagnostic applications, or research-grade research application.
- Display lot numbers and expiration dates to ensure traceability and quality control.
- Exclude any language suggesting safety, effectiveness, or clinical benefits in humans.
These labeling elements are essential compliance cornerstones that safeguard the distribution and use environment of Sermorelin as a research peptide.

Marketing and Advertising Limitations
The FDA strictly prohibits marketing RUO peptides such as Sermorelin with claims that imply research-grade or diagnostic utility. This means no online listings, brochures, or other promotional materials may state or infer that Sermorelin has been investigated for its effects on GH-related research deficiency, aging-related research, or any medical condition.
Additionally, direct-to-research subject advertising for RUO peptides is forbidden. Communications should be directed only to qualified researchers, clinicians, or licensed professionals familiar with compliance parameters. Violating these limitations risks regulatory action, including product seizures or warning letters. Research into Sermorelin research peptide continues to expand.
Best Practices for White-Label and Dropshipping Peptide Brands
For health and wellness clinics or entrepreneurs launching their own RUO peptide brands, maintaining strict FDA compliance is crucial to avoid legal pitfalls and protect business reputation. Consider these recommended practices: Research into Sermorelin research peptide continues to expand.
- Ensure all product packaging carries accurate RUO labeling: Work with suppliers or turnkey providers who print labels on demand that include mandatory statements, lot numbers, and expiration dates.
- Use disclaimers prominently in all marketing collateral: Make clear that products are research grade only, and that no medical claims are being made.
- Restrict sales to licensed professionals or researchers: Verify buyer credentials and avoid selling RUO peptides directly to researchers.
- Implement quality control and traceability systems: Lot tracking has been examined in studies regarding regulatory audits and recalls if needed.
- Partner with compliant dropship suppliers or white-label platforms: Choose providers that understand FDA rules and supply legal documentation to back compliance efforts.
Following these guidelines safeguards your brand, cultivates trust with professional clients, and ensures ethical handling of peptides like Sermorelin. At YourPeptideBrand (YPB), we specialize in helping clinics seamlessly integrate FDA-compliant peptide solutions — providing customizable, on-demand label printing, packaging, and direct dropshipping without minimum order restrictions to simplify your path into the RUO peptide market.
Business Opportunities with Sermorelin in the RUO Market
The rise of scientific wellness has opened lucrative avenues for health and wellness clinics aiming to integrate research-driven peptide products like Sermorelin into their service offerings. Sermorelin, with its robust scientific profile as a GH-related research-releasing hormone (GHRH) analog, presents a compelling entry point into the Research Use Only (RUO) market. Clinics can capitalize on this opportunity by developing educational and research-focused peptide lines that cater to the growing demand for innovative, science-backed health solutions.
One of the key advantages for clinics is the ability to differentiate themselves by offering peptides like Sermorelin under their own branded label, research examining credibility and research subject trust. By leveraging YourPeptideBrand’s turnkey white-label services, clinics can seamlessly launch and distribute customized peptide products without the typical logistical burdens. YPB’s on-demand custom labeling and packaging services eliminate the need for minimum order quantities or upfront inventory investment, significantly research examining effects on risk and financial exposure.
Additionally, YourPeptideBrand facilitates direct dropshipping to research subjects or individual clients, allowing clinics to expand their market reach effortlessly. This scalable distribution model enables practitioners to focus on research subject care and education while entrusting fulfillment and regulatory compliance to YPB’s experienced team. This integration of convenience and compliance streamlines the pathway to market entry, especially critical in the regulated peptide landscape.
Entering the RUO peptide space with Sermorelin offers substantial profitability potential. The peptide market is rapidly evolving, driven by consumer interest in scientifically validated wellness products and the growing acceptance of peptides as adjuncts in preventative health. By positioning Sermorelin as a foundational product within a clinic’s peptide portfolio, practitioners can attract a demographic eager for cutting-edge, research-supported options. This can not only boost research subject retention but also create recurring revenue streams from branded peptide sales.
To ensure successful and compliant brand launches, clinics must observe essential regulations governing RUO products. Key compliance checklist items include:
- Labeling Accuracy: Ensure all product labels clearly state “Research Use Only” and avoid research-grade claims to comply with FDA regulations.
- Marketing Practices: Marketing materials should emphasize peptide research and education without suggesting clinical research identification or research application.
- Storage and Handling: Maintain high standards for peptide storage, including cold chain management where necessary, to preserve product integrity.
- Documentation and Traceability: Keep thorough records of sourcing, batch testing, and distribution channels to establish transparency and regulatory adherence.
- Client Communication: Inform research subjects and end-research applications explicitly that RUO peptides are intended strictly for research purposes and not for human consumption or clinical use.
By aligning with these compliance best practices and leveraging YourPeptideBrand’s comprehensive white-label platform, health and wellness clinics can confidently launch their own Sermorelin-based product lines. This approach not only fosters innovation at the intersection of science and wellness but also cultivates a sustainable business model built on education, quality, and regulatory integrity.
Conclusion: Prudence and Potential in Sermorelin Research Use
Sermorelin remains a compelling subject of scientific interest due to its unique physiological role as a GH-related research-releasing hormone (GHRH) peptide analog. Unlike direct administration of GH-related research, Sermorelin mimics the natural pulsatile stimulation of the pituitary gland to release endogenous GH-related research. This distinction underpins its potential advantages in research contexts, as it has been examined in studies regarding a more physiologically aligned approach to studying GH-related research regulation and related biological effects.
It is crucial to reiterate that Sermorelin is currently designated strictly for Research Use Only (RUO). Our discussion and associated studies do not amount to endorsement of Sermorelin for research-grade or clinical use without regulatory approval. This classification underscores the importance of clear boundaries between exploratory research and direct research subject research application, ensuring compliance with FDA guidance and ethical standards.
The responsible adoption of Sermorelin in research settings demands informed decision-making, underscored by rigorous scientific scrutiny and regulatory awareness. Researchers and health practitioners must prioritize methodologies that respect existing frameworks, emphasizing safety, efficacy, and transparency. This prudent approach safeguards both the integrity of scientific inquiry and the well-being of individuals potentially impacted by any downstream therapies.
YourPeptideBrand (YPB) is dedicated to facilitating this rigorous, compliant engagement with peptides like Sermorelin. By providing a comprehensive suite of white-label, turnkey peptide branding and distribution solutions, YPB empowers clinics, healthcare professionals, and entrepreneurial ventures to participate confidently in peptide research markets. Our services encompass custom packaging, on-demand label printing, and direct dropshipping without minimum order constraints — all designed to align with FDA compliance and best practices in Research Use Only product development.
Clinics and wellness providers interested in exploring peptide research can leverage YourPeptideBrand’s expertise to establish their own research-focused branding initiatives. This not only has been examined in studies regarding scientific advancement but also opens avenues for sustainable business growth within a well-regulated framework. By adhering to ethical standards and maintaining laser focus on research applications, the peptide community can responsibly unlock Sermorelin’s scientific potential.
References and Source Documentation
For practitioners and entrepreneurs engaged with Research Use Only (RUO) peptides like Sermorelin, navigating regulatory frameworks and scientific literature is essential. Below are key authoritative resources to support compliance, deepen understanding, and inform best practices.
- FDA RUO Peptide Guidance: The U.S. Food and Drug Administration provides comprehensive regulatory guidance on Research Use Only products, including peptides. This official resource outlines compliance expectations crucial for clinicians and businesses involved in peptide distribution and usage.
- National Institutes of Health PubMed↗ Repository: PubMed hosts an extensive collection of peer-reviewed studies covering Sermorelin’s pharmacology, clinical applications, and mechanistic insights. This repository is invaluable for staying abreast of evolving research on GH-related research-releasing hormone (GHRH) analogs and their research-grade potential.
- Wikipedia Overview: For a concise yet informative summary of Sermorelin’s biochemical characteristics, approved uses, and pharmacodynamics, the Wikipedia entry serves as a convenient quick reference, backed by citations from primary literature.
- Key Peptide Pharmacology Texts: Standard pharmacology textbooks and recent comprehensive reviews on GHRH analogs and GH-related research replacement research application provide foundational knowledge and clinical context. These sources critically analyze the pharmacokinetics, safety profiles, and comparative efficacy of peptides like Sermorelin, research examining evidence-based application.
Among frequently cited references in this domain are Goodman & Gilman’s The Pharmacological Basis of Therapeutics and contemporary review articles in journals such as Endocrine Reviews and The Journal of Clinical Endocrinology & Metabolism. Practitioners are encouraged to consult these texts to deepen their grasp of peptide science underpinning Sermorelin’s role as a safer, physiologic alternative to direct GH-related research research application.
Utilizing these authoritative sources ensures that medical professionals and wellness clinics operate with scientific rigor and regulatory compliance when incorporating Sermorelin and related peptides into their offerings.
Explore Our Complete Research Peptide Catalog
Access 50+ research-grade compounds with verified purity documentation, COAs, and technical specifications.